
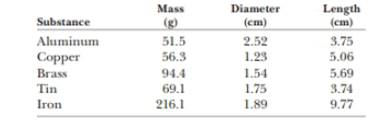
The data in the following table represent measurements of the masses and dimensions of solid cylinders of aluminum, copper, brass, tin, and iron. (a) Use these data to calculate the densities of these substances. (b) State how your results compare with those given in Table 14.1.
(a)
The densities of each substance.
Answer to Problem 1.61AP
The density of aluminum solid cylinders is
Explanation of Solution
Given info: The mass, diameter and length of each substance are given below,
| Substance | Mass
| Diameter
| Length
|
| Aluminum |
|
|
|
| Copper |
|
|
|
| Brass |
|
|
|
| Tin |
|
|
|
| Iron |
|
|
|
Formula to calculate the density of substance is,
Here,
Write the expression for the volume of solid cylinder,
Here,
Substitute
For aluminum:
Substitute
Thus, the density of aluminum solid cylinders is
For copper:
Substitute
Thus, the density of copper solid cylinders is
For brass:
Substitute
Thus, the density of brass solid cylinders is
For tin:
Substitute
Thus, the density of tin solid cylinders is
For iron:
Substitute
Thus, the density of iron solid cylinders is
Conclusion:
Therefore, the density of aluminum solid cylinders is
(b)
The comparison between results of part (a) and table
Answer to Problem 1.61AP
The density of aluminum from table is
Explanation of Solution
Given info:
Formula to calculate the percentage error is,
Here,
For aluminum:
From part (a), the density of the aluminum is
Substitute
Thus, the density of aluminum from table is
For copper:
From part (a), the density of the copper is
Substitute
Thus, the density of copper from table is
For brass:
From part (a), the density of the brass is
Substitute
Thus, the density of brass from table is
For tin:
From part (a), the density of the tin is
Substitute
Thus, the density of tin from table is
For iron:
From part (a), the density of the iron is
Substitute
Thus, the density of iron from table is
Conclusion:
Therefore, the density of aluminum from table is
Want to see more full solutions like this?
Chapter 1 Solutions
Physics for Scientists and Engineers, Technology Update (No access codes included)
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
Physics: Principles with Applications
College Physics
Cosmic Perspective Fundamentals
Essential University Physics (3rd Edition)
- A hollow spherical container has an outer diameter of 10.50 cm. The thickness of the walls is 0.50 cm. The container is filled with water. Water molecules are approximated to be spheres with a diameter of 275 pm. How many water molecules are present inside of the container? Your answer needs to have the correct number of significant figures.arrow_forwardEvery day, Jill collects one full bucket of water from the well up the hill. The well has a diameter of 0.800 m and a depth of 3.00 m. Currently, only 65.3% of the well is full. If Jill collects one full bucket every day from today, and it takes her 851 days to empty the well, how many pints of water was she collecting per trip? 1 qt = 2 pints, 1 L = 1.06 qt, 1 m3 = 1000 L, V = πr2harrow_forwardQuestion: Lets think about the pressure difference for bubbles in soda. If the bubble has a radius of 1 um and a surface tension of 0.072 N/m what is the pressure difference between the bubble and the soda? Give your answer in 3 significant figures. Solution: The pressure difference is Paarrow_forward
- Assuming biological substances are 100% water, estimate the mass of a fly. Take a fly to be roughly a cylinder 4 mm long and 2 mm in diameter. kgarrow_forwardThe U.S. Mint produces a dollar coin called the American Silver Eagle that is made of nearly pure silver. This coin has a diameter of 41 mmmm and a thickness of 2.5 mmmm. The density and approximate market price of silver are 10.5 g/cm3g/cm3 and $0.58 per gram, respectively. Calculate the value of the silver in the coin, assuming its thickness is uniform. Express your answer in dollars to the nearest dollar.arrow_forwardAn iron casting containing a number of cavities weighs 5.20E+3 N in air and 4.10E+3 N in water. What is the total volume of all the cavities in the casting? The density of iron (that is, a sample with no cavities) is 7.87 g/cm3. Number____ units:arrow_forward
- Juno is a construction worker. He has found a scrap piece of construction aluminum foil. The sheet has the dimensions of 1.00ft x 5.00ft. Juno knows the 3 aluminum density is 2. 70g/cm. He weighs it and finds its mass is 20.1 grams. Help Juno figure out how thick the sheet is. write your answer in millimeters and with the correct amount of significant figures.arrow_forwardRead the volume of the liquid in the Erlenmeyer flask. Estimate the volume to the nearest 5 mL increment and enter it with the proper number of significant figures. volume: volume: ml Ű Read the volume of the liquid in the beaker. Estimate the volume to the nearest 5 mL increment and enter it with the proper number of significant figures. Show Transcribed Text mL 100 Ċ M 200 ml 150 30 100 50 100 50arrow_forwardデジタル形式で段階的に解決 ありがとう!! SOLVE STEP BY STEP IN DIGITAL FORMAT Structure of Metal Materials: 3. The value of the theoretical density calculated for titanium can be calculated based on a rhomboidal arrangement, which represents one third of the compact hexagonal cell. Will the result obtained be valid for the complete or total compact hexagonal structure, which in total contains 6 atoms per unit cell? Explain your answer.arrow_forward
- Invalid path. A light balloon is filled with 383 m3 of helium at atmospheric pressure. (a) At 0°C, the balloon can lift a payload of what mass? kg (b) In the table below, observe that the density of hydrogen is nearly half the density of helium. What load can the balloon lift if filled with hydrogen? kg Densities of Some Common Substances at Standard Temperature (0°C) and Pressure (Atmospheric) p(kg/m3) p(kg/m3) Substance Substance Air 1.29 Ice 0.917 X 103 7.86 X 103 11.3 X 10 13.6 X 10 Aluminum 2.70 X 103 Iron Benzene 0.879 X 103 Lead 8.92 X 103 Mercury Copper Ethyl alcohol 0.806 X 105 Oak 0.710 X 103 Fresh water 1.00 X 103 Oxygen gas Pine 1.43 Glycerin 1.26 X 103 0.373 X 103 Gold 19.3 X 10 Platinum 21.4 X 10 1.03 X 10 10.5 X 10 Helium gas 1.79 X 10-1 Seawater Hydrogen gas 8.99 X 10-2 Silverarrow_forwardIn the figure below, what is the pressure of the gas in the flask (in atm) if the barometer reads 693.4 torr? (Ah = 8.37 cm) oo Round your answer to 4 significant figures. Open end Gas Ah Note: Reference the SI prefixes and Conversion factors for non-SI units tables for additional information. Pressure = 0.9123 atm 0 x10 X olo Ar Barrow_forwardWhat is the pressure of the gas in the cylinder, in kPa (kiloPascal)? h = 0.620 m, Pmercury = 13,600 kg/m3, 1.0 atm = 1.00 x 105 Pa = 100 kPa, and g = 10.0 m/s?. Your answer needs to have 3 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement. Mercury. p = 13,600 kg/m² Penarrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





