
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN: 9781133104261
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1, Problem 13OQ
Figure OQ1.13 shows two vectors →D1 and →D2. Which of the possibilities (a) through (d) is the vector →D2−2→D1, or (e) is it none of them?
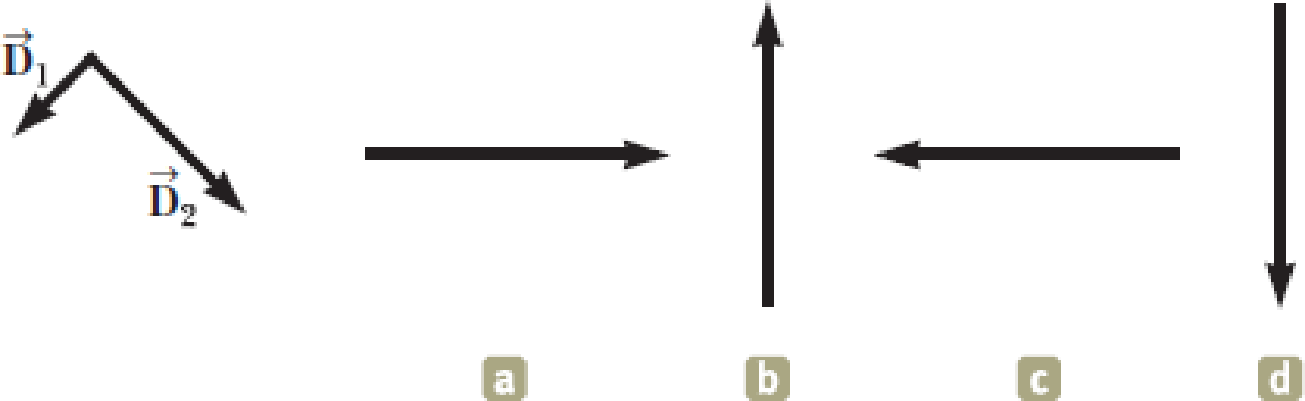
Figure OQ1.13
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I need help with part B. I cant seem to get the correct answer. Please walk me through what youre doing to get to the answer and what that could be
Question 6:
Chlorine is widely used to purify municipal water supplies and to treat swimming pool
waters. Suppose that the volume of a particular sample of Cl₂ gas is 8.70 L at 895 torr
and 24°C.
(a) How many grams of Cl₂ are in the sample?
⚫ Atomic mass of CI = 35.453 g/mol
• Molar mass of Cl₂ = 2 x 35.453 = 70.906 g/mol
Solution:
Use the Ideal Gas Law:
Step 1: Convert Given Values
• Pressure: P = 895 torr → atm
PV=
= nRT
1
P = 895 ×
= 1.1789 atm
760
•
Temperature: Convert to Kelvin:
T24273.15 = 297.15 K
• Gas constant: R = 0.0821 L atm/mol. K
Volume: V = 8.70 L
Step 2: Solve for n
.
PV
n =
RT
n =
(1.1789)(8.70)
(0.0821)(297.15)
10.25
n =
= 0.420 mol
24.405
Step 3: Calculate Mass of Cl₂
Final Answer: 29.78 g of Cl₂.
mass nx M
mass=
(0.420)(70.906)
mass=
29.78 g
E1
R₁
w
0.50
20 Ω
12
R₁₂
ww
ΒΩ
R₂
60
E3
C
RA
w
15 Ω
E2
0.25
E4
0.75 Ω
0.5 Ω
Chapter 1 Solutions
Principles of Physics: A Calculus-Based Text
Ch. 1.2 - True or False: Dimensional analysis can give you...Ch. 1.3 - The distance between two cities is 100 mi. What is...Ch. 1.7 - Which of the following are vector quantities and...Ch. 1.8 - Prob. 1.4QQCh. 1.8 - Prob. 1.5QQCh. 1.9 - Choose the correct response to make the sentence...Ch. 1.9 - If at least one component of a vector is a...Ch. 1.9 - If A + B = 0, the corresponding components of the...Ch. 1 - Answer each question yes or no. Must two...Ch. 1 - The price of gasoline at a particular station is...
Ch. 1 - Rank the following five quantities in order from...Ch. 1 - What is the y component of the vector (3i8k) m/s?...Ch. 1 - Prob. 5OQCh. 1 - Prob. 6OQCh. 1 - One student uses a meterstick to measure the...Ch. 1 - Prob. 8OQCh. 1 - What is the x component of the vector shown in...Ch. 1 - What is the y component of the vector shown in...Ch. 1 - Prob. 11OQCh. 1 - Prob. 12OQCh. 1 - Figure OQ1.13 shows two vectors D1 and D2. Which...Ch. 1 - A vector points from the origin into the second...Ch. 1 - Prob. 15OQCh. 1 - Prob. 16OQCh. 1 - Prob. 1CQCh. 1 - Prob. 2CQCh. 1 - Prob. 3CQCh. 1 - Prob. 4CQCh. 1 - A book is moved once around the perimeter of a...Ch. 1 - Prob. 6CQCh. 1 - Prob. 7CQCh. 1 - Prob. 8CQCh. 1 - Prob. 1PCh. 1 - Prob. 2PCh. 1 - Prob. 3PCh. 1 - Prob. 4PCh. 1 - Prob. 5PCh. 1 - Figure P1.6 shows a frustum of a cone. Match each...Ch. 1 - Prob. 7PCh. 1 - Prob. 8PCh. 1 - Prob. 9PCh. 1 - Prob. 10PCh. 1 - Prob. 11PCh. 1 - Prob. 12PCh. 1 - Prob. 13PCh. 1 - Let AI represent the density of aluminum and Fe...Ch. 1 - Assume it takes 7.00 min to fill a 30.0-gal...Ch. 1 - Prob. 16PCh. 1 - Find the order of magnitude of the number of...Ch. 1 - Prob. 18PCh. 1 - To an order of magnitude, how many piano tuners...Ch. 1 - Prob. 20PCh. 1 - Prob. 21PCh. 1 - Carry out the arithmetic operations (a) the sum of...Ch. 1 - Prob. 23PCh. 1 - Prob. 24PCh. 1 - A sidewalk is to be constructed around a swimming...Ch. 1 - Prob. 26PCh. 1 - Prob. 27PCh. 1 - Prob. 28PCh. 1 - Prob. 29PCh. 1 - Two points in the xy plane have Cartesian...Ch. 1 - The polar coordinates of a point are r = 5.50 m...Ch. 1 - Prob. 32PCh. 1 - Prob. 33PCh. 1 - Prob. 35PCh. 1 - A plane flies from base camp to Lake A, 280 km...Ch. 1 - A roller-coaster car moves 200 ft horizontally and...Ch. 1 - Prob. 38PCh. 1 - Prob. 39PCh. 1 - Find the horizontal and vertical components of the...Ch. 1 - Prob. 41PCh. 1 - Vector B has x, y, and z components of 4.00, 6.00,...Ch. 1 - Prob. 43PCh. 1 - Three displacement vectors of a croquet ball are...Ch. 1 - Prob. 45PCh. 1 - Vector A has x and y components of 8.70 cm and...Ch. 1 - Prob. 47PCh. 1 - Use the component method to add the vectors A and...Ch. 1 - Prob. 49PCh. 1 - Consider the three displacement vectors A=(3i3j)m,...Ch. 1 - A person going for a walk follows the path shown...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Prob. 55PCh. 1 - The distance from the Sun to the nearest star is...Ch. 1 - Prob. 57PCh. 1 - Prob. 58PCh. 1 - Prob. 59PCh. 1 - Prob. 60PCh. 1 - Prob. 61PCh. 1 - Prob. 62PCh. 1 - Prob. 63PCh. 1 - Prob. 64PCh. 1 - A child loves to watch as you fill a transparent...Ch. 1 - One cubic centimeter of water has a mass of 1.00 ...Ch. 1 - Prob. 67PCh. 1 - Prob. 68PCh. 1 - A pirate has buried his treasure on an island with...Ch. 1 - Prob. 70PCh. 1 - Prob. 71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the force (in N) on the 2.0 μC charge placed at the center of the square shown below? (Express your answer in vector form.) 5.0 με 4.0 με 2.0 με + 1.0 m 1.0 m -40 με 2.0 μCarrow_forwardWhat is the force (in N) on the 5.4 µC charge shown below? (Express your answer in vector form.) −3.1 µC5.4 µC9.2 µC6.4 µCarrow_forwardAn ideal gas in a sealed container starts out at a pressure of 8900 N/m2 and a volume of 5.7 m3. If the gas expands to a volume of 6.3 m3 while the pressure is held constant (still at 8900 N/m2), how much work is done by the gas? Give your answer as the number of Joules.arrow_forward
- The outside temperature is 25 °C. A heat engine operates in the environment (Tc = 25 °C) at 50% efficiency. How hot does it need to get the high temperature up to in Celsius?arrow_forwardGas is compressed in a cylinder creating 31 Joules of work on the gas during the isothermal process. How much heat flows from the gas into the cylinder in Joules?arrow_forwardThe heat engine gives 1100 Joules of energy of high temperature from the burning gasoline by exhausting 750 Joules to low-temperature . What is the efficiency of this heat engine in a percentage?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning
Components of a Vector (Part 1) | Unit Vectors | Don't Memorise; Author: Don't Memorise;https://www.youtube.com/watch?v=fwMUELxZ0Pw;License: Standard YouTube License, CC-BY
02 - Learn Unit Conversions, Metric System & Scientific Notation in Chemistry & Physics; Author: Math and Science;https://www.youtube.com/watch?v=W_SMypXo7tc;License: Standard Youtube License