
The predicted normal boiling point of the particular organic chemical should be determined.
Answer to Problem 1.29P
Explanation of Solution
Given:
Laboratory Data for the particular organic chemical.
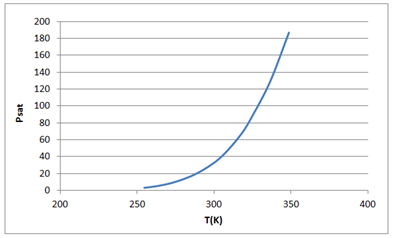
| T (C) | T (K) | Psat |
| -18.5 | 254.65 | 3.18 |
| -9.5 | 263.65 | 5.48 |
| 0.2 | 273.35 | 9.45 |
| 11.8 | 284.95 | 16.9 |
| 23.1 | 296.25 | 28.2 |
| 32.7 | 305.85 | 41.9 |
| 44.4 | 317.55 | 66.6 |
| 52.1 | 325.25 | 89.5 |
| 63.3 | 336.45 | 129 |
| 75.5 | 348.65 | 187 |
Below is the Antoine equation:
Here Psatis the function of A, B, C and T
Now differentiating (1) w.r.t to A
Now differentiating (1) w.r.t to B
Now differentiating (1) w.r.t to B
As the data given:
| T (C) | T (K) | Psat |
| -18.5 | 254.65 | 3.18 |
| -9.5 | 263.65 | 5.48 |
| 0.2 | 273.35 | 9.45 |
| 11.8 | 284.95 | 16.9 |
| 23.1 | 296.25 | 28.2 |
| 32.7 | 305.85 | 41.9 |
| 44.4 | 317.55 | 66.6 |
| 52.1 | 325.25 | 89.5 |
| 63.3 | 336.45 | 129 |
| 75.5 | 348.65 | 187 |
Now drawing the graph between Psat vs T as follows:
Using the MADCAD genfit function to fit the above data as follows:
Here a0 = A, a1 = B and a2 = C
Take the initial guess as
Now using the genfit function as follows:
The solution is
Thus, the fitted curve is
Calculating the values of function F as follows:
| T (C) | T (K) | Psat | (A-B)/(T+C) | F (A, B, C, T) |
| -18.5 | 254.65 | 3.18 | 1.081638 | 2.949506685 |
| -9.5 | 263.65 | 5.48 | 1.652271 | 5.218820016 |
| 0.2 | 273.35 | 9.45 | 2.210994 | 9.124778606 |
| 11.8 | 284.95 | 16.9 | 2.813238 | 16.6637818 |
| 23.1 | 296.25 | 28.2 | 3.340775 | 28.24099506 |
| 32.7 | 305.85 | 41.9 | 3.749392 | 42.49523245 |
| 44.4 | 317.55 | 66.6 | 4.204708 | 67.00104723 |
| 52.1 | 325.25 | 89.5 | 4.481671 | 88.38219805 |
| 63.3 | 336.45 | 129 | 4.856051 | 128.5156723 |
| 75.5 | 348.65 | 187 | 5.229728 | 186.742037 |
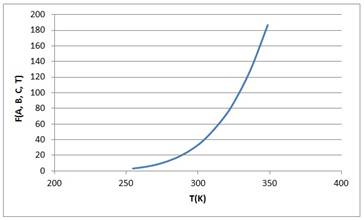
Hence the above graph is fitted with the data graph and thus the correct values of A, B an C are
Now calculating the normal boiling point by substituting Psat = 1 atm
Therefore, the normal boiling point is
Want to see more full solutions like this?
Chapter 1 Solutions
Introduction to Chemical Engineering Thermodynamics
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





