
Concept explainers
(a)
Interpretation:
Whether the following change is physical or chemical is to be determined.
Concept introduction:
The change that takes place only in state or appearance and not in the composition is known as physical change. The atoms or the molecules of a substance do not change their identity when a substance undergoes a physical change. The change accompanied by the change in the physical properties only is classified as physical change. The substance remains the same before and after the change. For example, the melting of ice is a physical change.
The change that takes place in the composition is known as chemical change. The atoms or the molecules of the substance rearrange and transformed into a new substance. For example, the burning of paper is a chemical change.
(a)
Answer to Problem 1.1P
The mixing of substances in A and B to give substance in C is a chemical change.
Explanation of Solution
The change is depicted as follows:
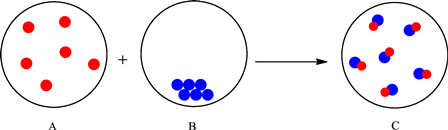
Each sphere represents one particle or atom. Atoms from A react with the atoms in B to form a new substance with one red and one blue atom depicted in the C. Formation of a new substance in a change is classified as a chemical change. Thus, the mixing of substances in A and B to give substance in C is a chemical change.
The particles in the A interact with the particles in B and result in the formation of new substance (change in composition). Therefore, it can be classified as a chemical change.
(b)
Interpretation:
Whether the following change is physical or chemical is to be determined.
Concept introduction:
The change that takes place only in state or appearance and not in the composition is known as physical change. The atoms or the molecules of a substance do not change their identity when a substance undergoes a physical change. The change accompanied by the change in the physical properties only is classified as physical change. The substance remains the same before and after the change. For example, the melting of ice is a physical change.
The change that takes place in the composition is known as chemical change. The atoms or the molecules of the substance rearrange and transformed into a new substance. For example, the burning of paper is a chemical change.
(b)
Answer to Problem 1.1P
The mixing of substances in A and B to give substance in D is a chemical change.
Explanation of Solution
The change is depicted as follows:
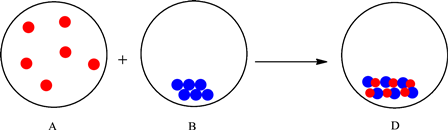
Each sphere represents one particle or atom. Atoms from A react with the atoms in B to form a new substance with one red and one blue atom depicted in the D. Formation of a new substance in a change is classified as a chemical change. Thus, the change is classified as a physical change. Therefore, the mixing of substances in A and B to give substance in D is a chemical change.
The particles in A interact with the particles in B and result in the formation of new substance (change in composition). Therefore, it can be classified as a chemical change.
(c)
Interpretation:
Whether the following change is physical or chemical is to be determined.
Concept introduction:
The change that takes place only in state or appearance and not in the composition is known as physical change. The atoms or the molecules of a substance do not change their identity when a substance undergoes a physical change. The change accompanied by the change in the physical properties only is classified as physical change. The substance remains the same before and after the change. For example, the melting of ice is a physical change.
The change that takes place in the composition is known as chemical change. The atoms or the molecules of the substance rearrange and transformed into a new substance. For example, the burning of paper is a chemical change.
(c)
Answer to Problem 1.1P
The conversion of substance C into D is a physical change.
Explanation of Solution
The change is depicted as follows:
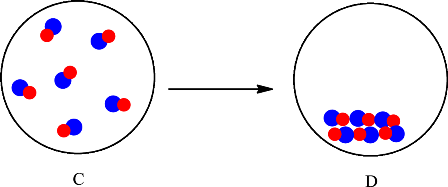
Each sphere represents one particle or atom. C consists of molecules made up of one red sphere and one blue sphere. D also consists of molecules made up of one red sphere and one blue sphere. The only difference is in the arrangement of the particles in C and D. In C the particles are far apart from each other and are in the gaseous state whereas in D the particles are arranged in a regular pattern and in the solid state. Since no new substance is formed, therefore conversion of substance C into D is considered as a physical change.
The particles in C rearranged to give substance D. Since no new substance is formed the change is classified as a physical change.
(d)
Interpretation:
Whether the following change is accompanied by the change in physical properties or chemical properties is to be determined.
Concept introduction:
The change that takes place only in state or appearance and not in the composition is known as physical change. The atoms or the molecules of a substance do not change their identity when a substance undergoes a physical change. The change accompanied by the change in the physical properties only is classified as physical change. The substance remains the same before and after the change. For example, the melting of ice is a physical change.
The change that takes place in the composition is known as chemical change. The atoms or the molecules of the substance rearrange and transformed into a new substance. For example, the burning of paper is a chemical change.
(d)
Answer to Problem 1.1P
After the change in part (c) has occurred the sample have different physical properties.
Explanation of Solution
The change is depicted as follows:
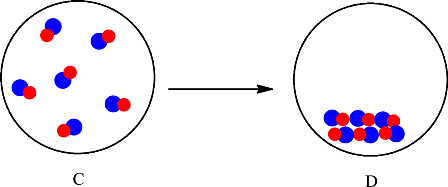
Each sphere represents one particle or atom. C consists of molecules made up of one red sphere and one blue sphere. D also consists of molecules made up of one red sphere and one blue sphere. The only difference is in the arrangement of the particles in C and D. In C the particles are far apart from each other and are in the gaseous state whereas in D the particles are arranged in a regular pattern and in the solid state. Since no new substance is formed, therefore conversion of substance C into D is considered as a physical change.
The change is physical change, therefore, substance C and D will have the same chemical properties but different physical properties.
The particles in C rearranged to give substance D. Since no new substance is formed the substance C and D are the same therefore they will have the same chemical properties but different physical properties.
Want to see more full solutions like this?
Chapter 1 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





