
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
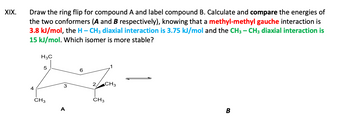
Transcribed Image Text:XIX.
Draw the ring flip for compound A and label compound B. Calculate and compare the energies of
the two conformers (A and B respectively), knowing that a methyl-methyl gauche interaction is
3.8 kJ/mol, the H-CH3 diaxial interaction is 3.75 kJ/mol and the CH3 - CH3 diaxial interaction is
15 kJ/mol. Which isomer is more stable?
4
H3C
5
CH3
A
6
2 CH3
CH3
B
Expert Solution
arrow_forward
Step 1
Lower energy of a conformer leads to more stable in compare to higher energy conformer.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6) Place a circle around the molecules below that are enantiomers of molecule 3 and squares around the molecules that are diastereomers of molecule 3. NH2 H₂N H₂N NH2 NH2 NH2 molecule 3arrow_forward2. Draw the two chair conformations for both cis- and trans-2-isopropylcyclohexanol (a planar form is shown below without specifying cis or trans). For each isomer, circle the lower energy chair conformation and also indicate which isomer, the cis or the trans, is the more stable isomer. ОНarrow_forwardDraw a pair of cis/trans stereoisomers with a molecular formula of C7H14. Label the cis compound and the trans compoundarrow_forward
- S T B Q The following compound exhibits two chair conformations. Draw them and indicate which one is more stable. Explain. H H CH3 H3C H3C CH3 Draw structures of nine isomers of C-He Identify the two isomers that are mirror images. Aarrow_forwardShown are two stereo representations of lactic acid. Compare the two structures shown. Determine whether they represent identical molecules or mirror images. СООН COOH HO. CH3 H3C H Determine the absolute configuration of the two structures. Left: O Right:arrow_forwardHN- H3C HN -NH "CH3 OH H. CH3 H,C. Br H3C CH3 CH3 HO. Br HO,arrow_forward
- Compare the two possible chair conformations of this molecule. Using A-values, calculate the difference in energy of the two possible chair conformations. H3C H3C Table of A-Values in kcal/mol -CH3 1.7 -ОН 0.87 -CH2CH3 1.75 -OCH3 0.67 -CH(CH3)2 2.15 -CI 0.48 -C(CH3)3 4.9 -Br 0.43arrow_forwardH8.arrow_forward40. Beginning with the Newman projection below as number 1, draw each of the six conformations for the molecule. Keep the back carbon static and rotate the front one clockwise. Number each drawing and rank them from the most stable to the least stable. Br H, Brarrow_forward
- There are four isomers of the compound shown below. Draw the chair conformation of each of the four isomers and its conformation isomers. Draw the most stable of the conformation on the left. Be sure your positioning of groups is clear.arrow_forwardWhat is the relationship (same, enantiomers, or diastereomers) between the following two molecules? You should find the absolute configuration at the stereo center to answer this question. H CH... HS CH₂OH HOH₂C H SHarrow_forward2. Assign the R or S configuration to each chiral center in the molecules below. CH2CH2B. CH3 .CO2H (H3C)3C. C=CH HO CH,F H,C=G+CH(CH,CH3)2 CH2CH,CH3 HO H C(CH3)3 ОН CH,CH,Br F"CH2Br 3 4 (numbers indicate the IUPAC numbering)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY