
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
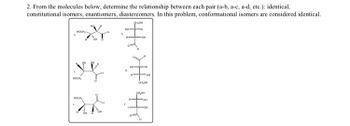
Transcribed Image Text:2. From the molecules below, determine the relationship between each pair (a-b, a-c, a-d, etc.): identical,
constitutional isomers, enantiomers, diastereomers. In this problem, conformational isomers are considered identical,
PUCH
H
HOCH
an e
OF CH
學
HOCH。
HOCH
CH H
OH
10-
h
H
d.
E
HO
CH
"OH
CH₂CH
PHON
美
FOH
H
-OH
Expert Solution
arrow_forward
Step 1z
Conformational isomers : (conformers): Isomers having the same bond connectivity sequence and can be interconverted by rotation around one or more single (σ) bonds.
Constitutional isomers: Constitutional isomers, also known as structural isomers, are specific types of isomers that share the same molecular formula but have different bonding atomic organization and bonding patterns.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following molecules, draw all possible stereoisomers using line-angle formulas with wedge/dash notation. Label the stereocenters as R or S. Indicate which ones are enantiomers and which ones are diastereomers. (Remember: there are 2 stereoisomers possible for a molecule with n chiral centers) (A) (B) (C) (1 chiral center) Br OH (2 chiral centers) CI OH CH3 (3 chiral centers)arrow_forwardUsing the drawing space below, draw the mirror image of the following molecule: CI H OH Br Once you have drawn the mirror image, decide whether your mirror image is an enantiomer of the molecule above, and click the yes or no button under the drawing area. Click and drag to start drawing a structure. Is your mirror image an enantiomer? O Yes Ο NO × × ●● Ś Śarrow_forwardWhat’s the main difference between enantiomers and diastereomers? I need help identifying the molecules.arrow_forward
- 7. The molecules shown are: I I A) B) C) D) E) IIIII 10 IOH Br CH 3 I identical. None of these HD Br Illll ITTIC constitutional isomers. enantiomers. diastereomers. OH ІН I CH3arrow_forwardUsing solid and dashed wedges, draw the structure of (1S, 2S, 4R, 5R)-1,5-dibromo-2,4- dichlorocyclohexane, then draw the most stable conformer.arrow_forwardSolve sub-part D,E, and Farrow_forward
- Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. Note that cis, trans isomers are an example of stereoisomers. НО. Br F Submit Answer OH F Br Br Retry Entire Group Br 1 more group attempt remainingarrow_forwardCompare the two possible chair conformations of this molecule. Given the AG for the two possible chair conformations, calculate the equilibrium constant for the interconversion between the two chair conformers at room temperature. CI H3C AG = 1.3 kcal/mol %3D AG = -RT In K %3D rearranges to K = e-AG/RT %3D R = 1.987 x 10-3 kcal/molK ||arrow_forwardWhich statement is correct about stereoisomers A, B, and C? H- CI CH3 S S CH3 A CI -H CI H A and B are enantiomers, C is a meso compound. A and C are enantiomers, B is a meso compound. A, B, and C are all meso compounds. Band C are enantiomers, A is a meso compound. CH3 R R CH3 B -H - CI H- H CH3 S R CH3 C CI -CIarrow_forward
- 15 eBook Print References 3 attempts left Check my work Select the single best answer. Identify the relationship in the following pair. Do the drawings represent constitutional isomers or stereoisomers, or are they just different ways of drawing the same compound? If they are stereoisomers, are they enantiomers or diastereomers? (select) H CH3 -Br and H -CH3arrow_forwardDoes the molecule below exist as a pair of enantiomers? If so, change the bonds to wedges and dashes to reflect R stereochemistry. If the molecule does not exist as a pair of enantiomers, check the box below. Does not exist as enantiomers OH 0 X H :0 Shmarrow_forwardDoes the molecule below exist as a pair of enantiomers? If so, change the bonds to wedges and dashes to reflect S stereochemistry. If the molecule does not exist as a pair of enantiomers, check the box below. H₂C- HO Does not exist as enantiomers X :0 Stmarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY