
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:A ALEKS-Alasia Fuqua-Learn
=
(43) THE NEW OUTLAST?!-Jaw X
← →
C www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-1BjuJnuCDtT6kRBabGFF3MOAKZ_UVXON202gQ_IWvS470tHNd1kBsKRoy8WfXEBm-Ky5RMDtaRgbulPm5W_Isl815AMZE_0?10Bw7QYJL <
O CHEMICAL REACTIONS
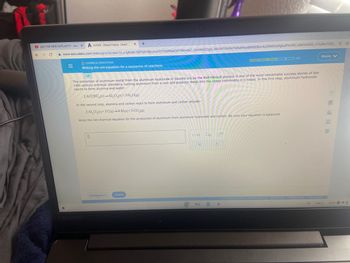
Writing the net equation for a sequence of reactions
X +
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late
19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide
reacts to form alumina and water:
2 Al(OH)3(s)-Al₂O3(s) + 3 H₂O(g)
11
In the second step, alumina and carbon react to form aluminum and carbon dioxide:
2 AL₂O3(s)+3 C(s)-4 Al(s) + 3 CO₂(9)
Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced.
Explagation
Check
THE 60
X
CD 3/5
M
D D
ES
Alasia V
Feb 2
F
allo
12:03 I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the smelting of iron ore which consists of Fe2O3 and Fe3O4. If Fe is produced in the pig iron, and FeO is produced in the slag, what are the chemical equations involved?arrow_forwardLooking up one snowy afternoon from a book titled The Moral Case Against Turning Lead Into Gold (Or Vice Versa), your friend Maria (an expert chemist) says this: "Metal sulfides roasted with oxygen produce the corresponding oxide and sulfur dioxide gas." Using Maria's statement, and what you already know about chemistry, predict the products of the following reaction. Be sure your chemical equation is balanced! NiS (s) + O₂(g) → 0-0 0+0 X 00 NO REACTION Sarrow_forwardReview Chemical reactions and Balancing Equations Complete and balanced the following reactions and state the type of reaction. (a) sodium + water → (b) magnesium chloride + ammonium hydroxide → (c) carbon + oxygen → (d) zinc + iron (II) sulfate → (e) sulfuric acid + calcium carbonate → (f) gold + copper (1I) chloride → (g) ammonium chloride + barium hydroxide → (h) butane + oxygen → (i) sodium sulfite + nitric acid → G) potassium carbonate → (k) beryllium chlorate → (1) magnesium + sulfuric acid > (m) lead (II) nitrate + potassium iodide → (n) sodium hydroxide + nitric acid →arrow_forward
- The Haber Process, developed by Fritz Haber in 1909, was a revolutionary method for producing ammonia from elemental hydrogen and nitrogen on an industrial scale. Write a balanced equation showing the conversion from elemental nitrogen (N2) and hydrogen (H2) to ammonia (NH3). (Omit states-of-matter from your answer.)arrow_forwardCryolite (Na3AlF6) is used in the commercial production of aluminum from ore. Cryolite itself is produced by the following reaction: 6 NaOH + Al₂O3 + 12 HF → 2 Na3AlF6 + 9 H₂O A mixture containing 420.0 kg of NaOH, 228.4 kg of Al2O3, and 600.0 kg of HF is heated to 950 °C until it reacts to completion. What is the maximum mass of Na3AlF6 formed? kg Na3AlF6arrow_forwardThe titanium chloride then reacts with liquid magnesium at 900°C to givetitanium and magnesium chloride (MgCl2). Write a balanced chemicalequation for this step in the refining of titanium.arrow_forward
- Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients.arrow_forwardAluminum is produced commercially by the electrolysis of Al2 O3 in the presence of a molten salt. If a plant has a continuous capacity of 1.49 million A, what mass of aluminum can be produced in 3.00 h? Mass = garrow_forwardQ2arrow_forward
- Balance the following chemical equations (include the states of matter) The hydrolysis of P4O10(s) with H2O(l) to produce phosphoric acid, H3PO4(l )arrow_forward21. Nitric acid is manufactured in the Oswald process, which involves the catalytic oxidation of ammonia according to the following equations: (i) 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g) (ii) 2 NO(g) + O2(8) 2 NO2(g); (iii) 3 NO2(g) + H2O(1) → 2 HNO3(aq) + NO(g); (NO is fed back into the second reactor) (a) The above equations may be combined to yield the following equation. Balance this equation. NH3(g) +_O2(g) + _H2O(1) _HNO3(1) + _H,O(1) + NO(g) (b) What is the mole fraction of NH3 that becomes nitric acid in one reaction cycle? (c) How many kilograms of HNO3 will be produced in one reaction cycle from 1.00 m³ of NH3, measured at STP, if the overall yield is 92.0%? (d) If concentrated nitric acid contains 70.0% (by mass) of HNO3 and the solution has density of 1.48 g/mL, how many litters of concentrated nitric acid is produced in this process at the above reaction yield? (Answers: (b) 2/3; (c) 1.73 kg of HNO3; (d) 1.67 L)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY