
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please don't provide handwriting solution
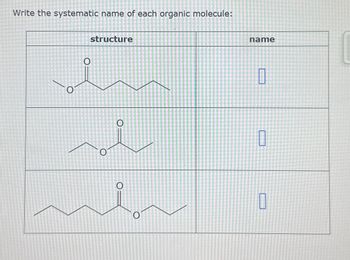
Transcribed Image Text:Write the systematic name of each organic molecule:
structure
in
سلمه
name
U
☐
Ο
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why is a salt solution used in the preparation of soap?arrow_forwardHow many kilograms (kg) of 20-20-20 soluble fertilizers should you add to a 50 gallon concentrated stock tank using and injector dilution ratio of 200:1 to provide a fertilizing solution of 200 mg N/L for plants plants?arrow_forwardGraph data with Concentration on the x-axis and Absorbance on the y-axis. Find the line of best fit for your data points. Record the respective formula in y=mx+b.arrow_forward
- Perform calculations to determine the amount of 5.00x10-5 M stock solution needed to prepare 20.00 mL of 2.00x10-5 M dye solution. Perform calculations to determine the amount of 2.00x10-5 M dye solution needed to prepare 20.00 mL of 5.00x10-6 M dye solution. Perform calculations to determine the amount of 5.00x10-6 M dye solution needed to prepare 20.00 mL of 1.00x10-6 M dye solution. Show your calculationsarrow_forwardDescribe the steps involved in recrystallization.arrow_forwardGive the name of material which is used for water purification.arrow_forward
- Suppose you measure a volume of 9.0 mL and a mass of 7.04 g for your first distillate. What is the density of the liquid? Group of answer choices 0.78 g/mL 0.782 g/mL 1.28 g/mL 1.3 g/mLarrow_forwardA student was given a stock ascorbic acid solution of 2.323 mg/L. Following the directions in the lab, the student obtained the data below. Calculate the concentration of ascorbic acid in the Standard (in microgram/L, ug -). Report your answer with one place after the decimal. initial final initial volume final volume volume volume color reagent color reagent stock stock solution solution solution solution Standard 1.35 mL 6.64 mL 5.12 mL 9.32 mLarrow_forwardDissolution Distilled H20 Concentration 0.01 M HCI Concentration (mg/ml) Time Total amount A Total amount A (mg/ml) (mg) (mg) 5 0.16 0.1 10 0.24 0.18 15 0.35 0.25 20 0.48 0.34 25 0.58 0.4 30 0.67 0.5 1. Plot the given data on an ordinary paper and find the slope for each line then calculate the IDR constant for aspirin in the two dissolution media, water and HCI. NOTE: Slope of the calibration curve = 1.0 (mg/ml and saturation solubility of salicylic acid is equal to 2.0 gm/L.arrow_forward
- Concentration of TZ stock solution (μMμM) 42.06arrow_forwardMost of us have had a glass of water from a Brita® pitcher. What is inside of a Brita® filter cartridge and what does it filter out? What doesn’t it filter out?arrow_forwardPlease give me answers in 5min I will give you like surearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY