
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please give me answers in 5min I will give you like sure

Transcribed Image Text:2. What is the molar extinction coefficient (e) for o-nitrophenol at max? Explain how you
arrived at this value.
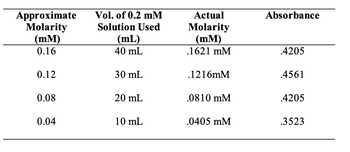
Transcribed Image Text:Approximate
Molarity
(mm)
0.16
0.12
0.08
0.04
Vol. of 0.2 mM
Solution Used
(mL)
40 mL
30 mL
20 mL
10 mL
Actual
Molarity
(mm)
.1621 mM
.1216mM
.0810 mM
.0405 mM
Absorbance
.4205
.4561
.4205
.3523
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- wileyplus.com/edugen/Iti/main.uni Return to Blackboard Malone/Hein, Basic Concepts of Chemistry, for Southwestern Illinois College Help | System Announcements Malone Problem 07-07 Iron rusts according to the equation 4Fe(s) + 30,(g) → 2Fe,0;(5). What mass of rust (Fe,0,) is formed from 0.330 mole of Fe? g Fe;03 the tolerance is +y-2% What mass of rust is formed from 0,330 mole O,7arrow_forwardCHM 431A Lab 7 - 2021SP Given Data page Time Temperatu (min) C1. Boiling temp of water re (°C ) 25.4 Initial temperature of water 1 32,5 Temperature of boiling water 44.9 3 56.9 C2. Temperature change (AT) 4 71.3 Mass of beaker 86.6 6 100.2 Mass of beaker and water 100.6 C3. Mass of water 8 100.6 9 100.6 Number of calories needed to heat your water to the boiling point: Heat (cal) = mass (g) x AT (°C) x 1.00 cal/g°C Show work Print out the graph paper, do the graph in pencil. Graph Time and Temperature relation of heating the water. Take a screen shot and submit your graph as attached file named Lab 7 pg. 4arrow_forwardAn infant acetaminophen suspension contains 80 mg/0.80 mL suspension. The recommended dose is 15 mg/kg body weight. How many milliliters of this suspension should be given to an infant weighing 14 LB? ( assume two significant figures.)arrow_forward
- Edit View History Bookmarks People Tab Window Help O Questi x L 2021-0 x M [EXTE X o Dashb x O Launci x O Launc x O Class 9 ba mheducation.com/ext/map/index.html?_con=con&external_browser3D0&launchUrl=https%253A%252F%252Fnewconn Problem Set Saved 1 attempts left Check my work Enter your answer in the provided box. Find AS for the combustion of ethane (C,H) to carbon dioxide and gaseous water. Report the entropy change per mole of ethane that undergoes combustion. J/(mol·K) < Prev 15 of 15 Nextarrow_forward2 1 20 Obey 1 1 11 1 11 tv 『 Answer Bank Violate 11 1 1 1 1 1 NiA@ zoomarrow_forwardIn an experiment, beams of neutral carbon atoms (C), C+ cations, and C anions fly from the left to the right between two metal plates, as shown in the figure. The top plate is connected to the positive terminal of a battery, while the bottom - to the negative terminal of the same battery. How will the electric field between the plates affect the trajectories of each type of particles: will they be deflected upward (trajectory 1), remain unaffected (trajectory 2), or be deflected downward (trajectory 3)? The C+, C, C- beams will follow trajectories 1,3,2 3,2,1 3,1,2 2, 3, 1 + 1, 2, 3 ---> respectively. 1 2 3arrow_forward
- Time, s PCH4, bar 0 1.01E+00 0.3559 7.48E-01 0.6817 5.98E-01 1.0074 4.98E-01 1.384 4.26E-01 1.6201 3.73E-01 2.0159 3.31E-01 2.3532 2.98E-01 2.7874 2.71E-01 3.0227 2.48E-01 3.3678 2.29E-01 A student collected the following data in the kinetics of the decomposition of methane at 1000K: Reaction: CH4(g)=> C(s) + H2(g) Build three appropriate Excel graphs and list the trendline equation and R2 for each including coordinates. The order of the reaction is? Justify your answer Report the reaction constant value with unitsarrow_forwardThe following arrows are incorrectly drawn and I drawn them correctly please check if it's right and write a little explanation how it's rightarrow_forward! A Escape Chemistry 106! → C Type here to search X + https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG4Oh2E11 120 1 2 3 4 Your answer Back 0.0154 M 0.0154 M 0.0308 M 0.0154 M This is a required question 0.0393 M 0.0131 M 0.0393 M 0.0393 M A decomposition reaction is determined to be zero order. The initial concentration of the reactant is 0.500 M. The rate constant for the reaction is 0.0000273 M/s. How long, in seconds, will it take for the concentration of the reactant to reach 0.378 M? Next Never submit passwords through Google Forms. 2:11 a 28 NuVCU4MS9 120% W 0.114 M 0.228 M 0.228 M 0.228 M 6.62 x 10-4 7.36 x 10-5 1.32 x 10-3 6.62 x 10-4 88 Ⓒ↓ |111\ Clear form 67°F J ABP 10:04 PM 5/11/2023arrow_forward
- rk for 07-CHEMISTR E Chem Test: The Mole (Conte C Meet - kkj-axdy-iic 20140108165311196.tif B Brainly.com - For students. A docs.google.com/forms/d/e/1FAlpQLSeL1Rc2BH6MExJbduVXpH6adz_Snn5TSSTYQIGIB7NL6dN02Q/formResponse M Gmail DpuTube A Maps Translate pnpbusinesscards Avery Print from th... photo shoot shirt A pure sample of an unknown molecule has a mass of 35.51 g and contains 0.2000 moles. What is the molecular (molar) mass of the substance? 177.55 g/mol O 7.102 g/mol O .0056 g/mol 5.63 g/molarrow_forward| ax NME /Chemical%20Reactions%20Asgn%202-5.pdf 9 Employee Home r4c Covenant Health Ca... Outlook.com - Free... S Birthday party politi. G what does it mea 1 / 2 100% Chemical Reactions-Write Neat and Balanced Reactions from Word Equations You need to submit one sheet of paper (not this one), that is very neat, with a fully balanced reaction for these 6 reactions Remember to include proper states. 1. solid potassium metal reacts with oxygen gas to produce solid potassium oxide. 2. solid potassium chlorate decomposes into oxygen gas and solid potassium chloride. 3. solid aluminum oxide is decomposed into solid aluminum and oxygen gas. 4. aqueous cobalt(III) nitrate reacts with solid zinc to produce aqueous zinc nitrate and solid cobalt.arrow_forwardΣ * 00 T The aromatic compound C6H-NH, is best-referred to as: O a. Phenol O b. Aniline O c. Toluidine Od. Toluene e. Nitrobernzene Time left 1:28:48 Quiz navigation DELL Esc F1 F2 F3 F4 F5 F6. F7 F8 F10 i V 3. 4. 5. 7. qel Lock C. Altarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY