
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
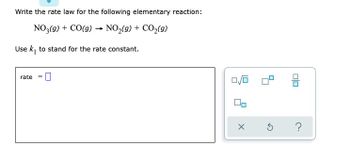
Transcribed Image Text:Write the rate law for the following elementary reaction:
NO3(g) + CO(g) → NO₂(g) + CO₂(g)
-
Use k₁ to stand for the rate constant.
rate =
0
0/0
00
X
010
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction, A + B + C => D, which is found to be first order in A, first order in B and first order in C. Which step of the proposed mechanism must be slow in order to agree with this rate law? 1. A(g) + B(g) => X(g) 2. X(g) + C(g) => Y(g) 3. Y(g) => D(g) A. 1 B. only 3 C. Either 2 or 3 D. only 2arrow_forwardWrite the rate law for the following elementary reaction: ICl(g) + H₂(g) HI(g) + HCl(g) Use k₁ to stand for the rate constant. 1 rate = 00 X Ś 00arrow_forwardWrite the rate law for the following elementary reaction: CFC13(9) CFC1₂(g) + Cl(g) Use k₁ to stand for the rate constant. rate 0 ->> 0/0 X ㅁ G 19arrow_forward
- The isomerization of cyclopropane, C3H6, is believed to occur by the mechanism shown in the equations above. Here C3H6* is an excited cyclopropane molecule. At low pressure, Step 1 is much slower than Step 2. Derive the rate law for this mechanism at low pressure?arrow_forwardInitial rate: 1.6 •10^-7mol/L•s K=6.4 •10^-9L/mol•s Rate law: k[HI]^2 Can you please show me the steps.arrow_forwardConsider the hypothetical reaction: A + B + 2C → 2D + E where the rate law is Rate = -ALA) = k[A][B]2 An experiment is carried out where [Alo = 7.200 x 102 M, [B]. = 2.500 M and [Cla = 2.500 M. The reaction is started, and after 11.60 seconds, the concentration of A is 2.200 x 10° M. Calculate the value of k for this reaction in units of M2s1. Answer:arrow_forward
- Click in the answer box to activate the palette. Enter the general form of the rate law for the following process. Represent the order as n, n', and so forth. Complete the equation using brackets, [], to represent concentration. 2Cu(g) + O2(g) → 2CuO(g)arrow_forwardConsider this reaction: 2HI (g) → H, (g) +I, (g) At a certain temperature it obeys this rate law. rate =(0,465 s)[HI Suppose a vessel contains HI at a concentration of 0.540M. Calculate the concentration of HI in the vessel 2.80 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits.arrow_forward0/5 Writing the rate law of an elementary reaction Write the rate law for the following elementary reaction: 2 NO(g) + Br,(g) + 2 NOBR(g) Use k, to stand for the rate constant. ratearrow_forward
- Nitrogen monoxide, NO, reacts with hydrogen to give nitrous oxide, N20, and water. 2NO(g) + H2 (g) → N½O(g) + H20(g) In a series of experiments, the following initial rates of disappearance of NO were obtained: Initial Concentration of NO Initial Concentration of H2 Initial Rate of Reaction of NO Exp. 1 8.6 x 10-3 M 4.1 x 10-3 M 8.7 x 10-5 M/s 3 Exp. 2 1.7 x 10-2 M 4.1 x 10- M 3.5 x 10-4 M/s Exp. 3 8.6 x 10-3 M 8.2 x 10-3 M -4 1.7 x 10 M/sarrow_forwardConsider this reaction: 2SO3 (g) →2SO₂ (g) + 0₂ (8) At a certain temperature it obeys this rate law. rate= (0.00626 s¹)[03] Suppose a vessel contains SO3 at a concentration of 0.250M. Calculate the concentration of SO3 in the vessel 99.0 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 X Śarrow_forwardWrite the rate law for the following elementary reaction: 2 N,05(9) → 2 N2O4(9) + O2(g) Use k, to stand for the rate constant. ratearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY