
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A possible mechanism for the reaction described by:
CO(g) + Cl2(g) → COCl2(g)
Identify any intermediate species + write the rate law for each step.
*I'm confused on how to identify intermediate species and why the rate law for 2 = k2[Cl]2
Answers: Cl(g) and COCl(g) are intermediate species. The rate laws are k1[Cl2], k2[Cl]2, k3[Cl][CO], and k4[COCl][Cl2], respectively.
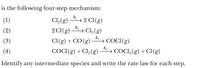
Transcribed Image Text:is the following four-step mechanism:
k,
Cl, (g)
→ 2 Cl(g)
→ Cl,(g)
(1)
(2)
k2
2 CI(g)
kg
Cl(g) + CO(g)
→ COCI(g)
→ COCI,(g) + Cl(g)
(3)
(4)
k4
COCI(g) + Cl2(g)
Identify any intermediate species and write the rate law for each step.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements about rate laws is FALSE? A) Rate laws can only be determined experimentally. B) Rate laws tell us information about the mechanism of the chemical reaction under study. C) The rate law for the chemical reaction A + B + 20 –D must be rate = k[A][B][C]² %3D D)A rate law can only be written for the elementary reactions of a given chemical reaction.arrow_forward4. The rate law for the reaction H₂ (g) + I₂ (g) → 2 HI (g) was determined to be rate = k [H₂] [1₂], which led to a simple single-step bimolecular mechanism where an H₂ molecule simply collided with an I₂ molecule. In the 1960s, though, different teams found spectroscopic evidence for this mechanism: (1) (2) (3) I₂ (g) →21 (g) H₂(g) + I (g) → H₂I (g) H₂I (g) + I (g) → 2 HI (g) (fast) (fast) (slow) Show that this mechanism is consistent with the rate law: rate = k [H₂] [1₂]. To do this, assign individual rate constants for ALL forward and reverse reactions in the mechanism (I'm guessing k₁, k-1, k2, k-2 and k3). Then show that the overall rate law from combining individual rate laws for the mechanism steps yields rate = (some combination of k's) [H₂] [1₂], and thus k= (some combination of k's). Remember that there should be no reaction intermediates in the rate law, only reactants. \arrow_forward16 redonearrow_forward
- Initial rate: 1.6 •10^-7mol/L•s K=6.4 •10^-9L/mol•s Rate law: k[HI]^2 Can you please show me the steps.arrow_forwardThe rate law for the reaction NO2(g) + O2(g) is given by rate = - k[NO₂] NO(g) + 03 (g) If the following is the mechanism for the reaction, which of the following statements correctly describe the reaction? Check all that apply. NO2(g) NO(g) + O(g) - O(g) + O2(g) 03 (g) The reaction is 2nd order overall. The first step is the slow step. Doubling NO, would quadruple the rate. Cutting O, in half would decrease the rate by a factor of 2. The molecularity of the first step is 1. Both steps are termolecular. None of the abovearrow_forwardConsider this reaction: 2SO3 (g) →2SO₂(g) + O₂(g) At a certain temperature it obeys this rate law. rate = (0.894 M¹-s¹) [S0₂] Suppose a vessel contains SO3 at a concentration of 0.390 M. Calculate the concentration of SO3 in the vessel 8.60 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M Explanation Check 0 10 Q Search a Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use O Privacy Centerarrow_forward
- 3. Consider the reaction below: 2NO (g) + Cl,(g)'n 2NOCI (g) A mechanism involving these steps has been proposed for the reaction: Step 1: NO (g) + Cl,(g)'n NOCI, (g) Step 2: NOCI, (g) + NO(g)'n 2NOCI (g) The rate law for the reaction is given by rate = k[NO][Cl]. (a) What is the reaction intermediate? (b) Which is the rate-determining step? (c) Is the rate-determining step unimolecular, bimolecular, or termolecular? Why?arrow_forwardClick in the answer box to activate the palette. Enter the general form of the rate law for the following process. Represent the order as n, n', and so forth. Complete the equation using brackets, [], to represent concentration. 2Cu(g) + O2(g) → 2CuO(g)arrow_forwardPlease complete the question typed ONLY. Please do not use handwriting.arrow_forward
- The exponents in a rate law describe the effects of the reactant concentrations on the reaction rate and define the reaction order. Consider a reaction for which the rate law is: rate = k[A]m[B]n If m is 1, the reaction is first order with respect to A. If the exponent is 2, the reaction is second order with respect to A. If n is 1, the reaction is first order in B. If m or n is zero, the reaction is zero order in A or B. If m = 1 and n = 1, the overall order of the reaction is second. (m + n = 1 + 1 = 2). 1. What is the difference between a reaction rate and a reaction's rate constant? 2. What does it mean if a reaction is zero order with respect to a reactant? 3. Doubling the concentration of a reactant increases the rate of a reaction four times. What is the order of the reaction with respect to that reactant? 4. Doubling the concentration of a reactant increases the rate of a reaction four times. Tripling the concentration of a different reactant increases the rate of a reaction…arrow_forwardThe reaction 2 NO(g) + O2(g) --> 2 NO2(g) proceeds through the following mechanism: 2 NO(g) --> N2O2(g)N2O2(g) + O2(g) --> 2 NO2(g) (a) The second step of this mechanism is rate-determining (slow). What is the rate law for this reaction? Rate = k [NO] [O2] Rate = k [NO]2 [O2] Rate = k [NO] [O2]2 Rate = k [NO]1/2 [O2] Rate = k [NO] [O2]1/2 Rate = k [NO]2 Rate = k [NO]2 [O2]1/2 (b) What would the rate law be if the first step of this mechanism were rate-determining? Rate = k [NO] [O2] Rate = k [NO]2 [O2] Rate = k [NO] [O2]2 Rate = k [NO]1/2 [O2] Rate = k [NO] [O2]1/2 Rate = k [NO]2 Rate = k [NO]2 [O2]1/2 Rate = k [NO]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY