
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ted
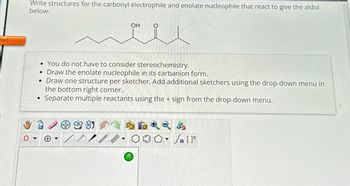
Write structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol
below.
●
• You do not have to consider stereochemistry.
●
Draw the enolate nucleophile in its carbanion form.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in
the bottom right corner.
Separate multiple reactants using the + sign from the drop-down menu.
****
8
OH
?
O
Sn [F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carefully draw the keto form of the compound for which the enol is drawn; then draw it's "enolate' formed by removing the acidic proton, and showing two resonance forms of it. 2. OH - H* keto form enol form enolate formsarrow_forwardArrange the following functional groups according to their DECREASING reactivity with nucleophiles. [esters, carboxylic acids, acyl halides, amides, anhydrides]arrow_forwardWhat is the name of the carbonyl compound that has served as common starting material for synthesis of each of the compounds in the box? NCH3 CH2OH H. A) acetaldehyde B) acetophenone C) benzaldehyde D) ethyl acetate A Вarrow_forward
- Complete the synthesis of ethyl (E)-2,2,4-trimethyl-3-oxo-5-phenylpent-4-enoate from the starting material given (ethyl propionate). You must list out all reagents/solvents next to the reaction arrows and draw the intermediate structures in the boxes. I am giving you one restriction. You are not allowed to introduce any enolates as reagents next to the reaction arrows. orff ionarrow_forwarddraw the product, show its sterochemistryarrow_forward1. Each of the following compounds can be prepared by a mixed aldol condensation reaction. Give the structures of the aldehyde and/or ketone precursors for each aldol product shown. soarrow_forward
- providing the chemical structure of the missing reactants or the products.arrow_forward5. In the transition state for the proline-catalyzed aldol addition, a hydrogen bond is shown between the carboxylic acid of proline and isobutyraldehyde. Explain how this could lower the energy barrier for the reaction.arrow_forwardWrite the structures of all the possible enolates for the following ketone. Label the kinetic and thermodynamic enolates. Indicate reagents and conditions (including solvent, temperature etc.) under which each product would predominate. Sketch a labelled reaction coordinate diagram indicating the progress of the formation of the enolates.arrow_forward
- 4. Please give the starting materials that would be necessary to make the following imines and enamines. a C d N. N atarrow_forwardShow how crossed Claisen condensations, malonic ester syntheses,acetoacetic ester syntheses, andRobinson annulations can synthesize a wide variety of ketone, ester,and acid derivatives.arrow_forwardWe have seen that the alpha carbon atom of an enamine can function as a nucleophile in a Michael reaction, and in fact, enamines can function as nucleophiles in a wide variety of reactions. For example, an enamine will undergo alkylation when treated with an alkyl halide. Draw the structure of intermediate A and the alkylation product B in the following reaction scheme (J. Am. Chem. Soc. 1954, 76, 2029-2030): TSOH -H₂O NH A 1) CHgI 2) H₂O+ B + NH Modify the given copy of the starting material to draw compound A. If needed, use the single bond tool to interconvert between double and single bonds. Edit Drawingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY