
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Write and balance this reaction: silver nitrate plus solid aluminum yields, write out the
products. Identify reaction type.
Remember to write states for each reactant and product.
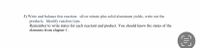
Transcribed Image Text:5) Write and balance this reaction: silver nitrate plus solid aluminum yields, write out the
products. Identify reaction type.
Remember to write states for each reactant and product. You should know the states of the
elements from chapter 1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe the characteristics of single replacement reaction, how it is identified, and what determines if this reaction will occur. Give an example in your response. (please write neatly and clearly)arrow_forward2. A double replacement reaction occurs between sodium hydroxide and copper(II)nitrate. a. Write the balanced equation for this reaction. (Note: Be sure to include the states of all reactants and products). b. Write the complete ionic equation for this reaction. (Note: Be sure to highlight the spectator ions in this reaction.) c. Write the net ionic equation for this reaction.arrow_forwardFor each question, do the following four things: 1. Predict the products that will be made in this double replacement reaction. 2. Write the formulas for each substance. 3. Record the states of matter. 4. Balance the equation.arrow_forward
- what is the skeleton equation for Magnesium metal burns in oxygen gas with a bright white light to make a white powder called magnesium oxide.arrow_forward2) Write and balance this reaction: solid iron (III) oxide produces solid iron plus oxygen gas. Identify reaction type. Remember to write states for each reactant and product. You should know the states of the elements from chapter 1.arrow_forwardWrite the balanced equation for the following situation. In addition, list the reaction type. You must tell the amount of every substance that remains in the container at the end of the reaction. Assume that all reactions go to completion. If only stoichiometry, tell how much excess reactant is used. Reaction Type:a. Combination Reactionb. Decomposition Reactionc. Single Displacement / THIS IS ONE TYPE OF Oxidation Reduction Reaction d. Precipitation Reactione. Gaseous Reactionf. Neutralization Reactiong. Combustion Reaction 61.802 cg of nitrogen gas is reacted with 61.802 cg of hydrogen gas to form ammoniaarrow_forward
- Complete and balance the following reactions - assume they all go a. hydrogen gas plus nitrogen gasb. potassium metal plus sulfur powderc. solid magnesium oxide plus waterarrow_forwardWhat type of chemical reaction may involve a metal atom transferring one or more electrons to a metal cation, producing a new metal atom and a new metal cation? a) double replacement b) single replacement c) synthesis d) decompositionarrow_forwardFor the following, (1) predict the products, (2) write the balanced equation with formulas, (3) determine if it is a synthesis, decomposition or combustion reaction.arrow_forward
- A solution of zinc acetate and a piece of aluminum metal are mixed together in a test tube. For the following reaction, a) Determine the type of reaction, and bi) if the reaction occurs, write the balanced reaction, including all states of matter, or bii) if the reaction does not occur, explain why the reaction does not occur.arrow_forwardOne day in lab, while adding a gnarled root to a dark liquid bubbling in an iron cauldron, your friend Raina (an expert chemist) says this: "Many metals can be produced from their oxide ores by reaction at high temperatures with carbon monoxide. Carbon dioxide is a byproduct." Using Raina's statement, and what you already know about chemistry, predict the products of the following reaction. Be sure your chemical equation is balanced! CoO(s) + CO(g) ->arrow_forwardWrite and balance this reaction: sodium plus nitrogen produces solid sodium nitride. Remember to write states for each reactant and product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY