
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

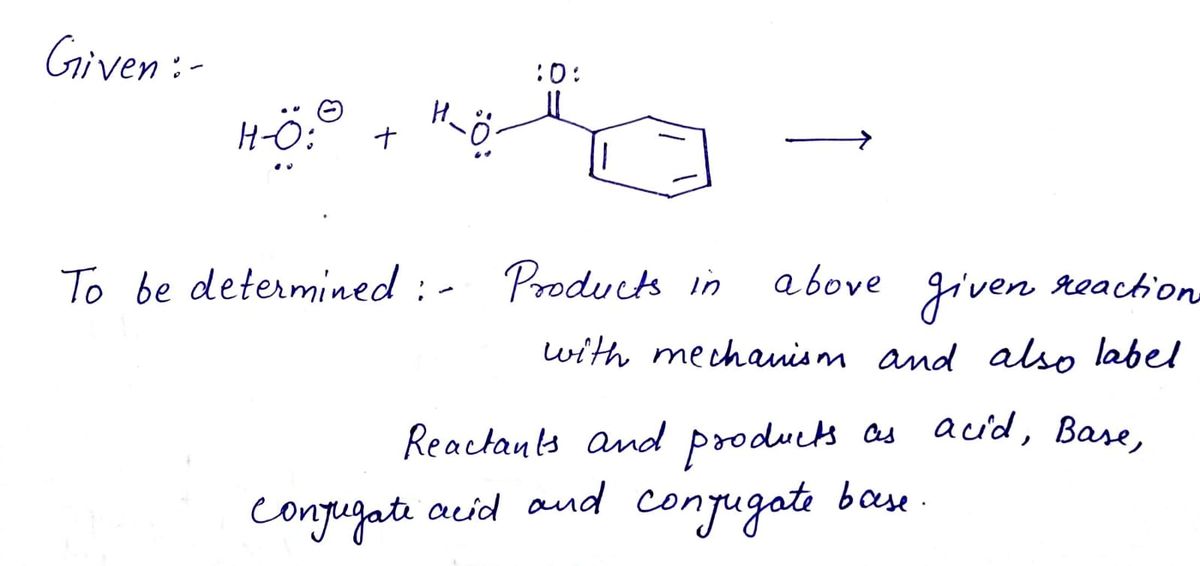
Transcribed Image Text:Write a mechanism, using curved arrows, for the acid-base reaction given below. Show the
correct structures for the products (including lone pair electrons). Label each component as
acid, base, conjugate acid, and conjugate base.
:o:
H..:
H-C
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 8:01 Back Module 3 Homework.docx that structure affects acranty, with a greater degree of resonance, the conjugate base becomes more stabilized, meaning that the acid form is more acidic. Draw appropriate resonance structures to show why one compound in the pair is more acidic. F₂C OH Bond Strength and its effects on acidity HSH HO Rank the following acids in order of increasing acidity (1 being the least acidic) and support each answer, briefly. Observed Trend: HF OH pka = 7 pka 15.7 Trends can be seen within the periodic table that can dictate acidity based on bond. strength.; all things being equal, the stronger the bond the weaker the acid. HCI Additional Problems Dashboard Calendar H₂C OH HBr weakest acid strongest acid Explain this observed trend based on bond strength and bond length. To Do HI Show by writing appropriate resonance structures that the two compounds shown form the same conjugate base upon innination Whinh atam in the naminaata hana Douf haava 44 Notifications MeOH…arrow_forwardArrange the acids below in order of predicted decreasing strength (i.e., from strongest to weakest). A H3NB HClC H3PD H2SE H3Asarrow_forward2. a) For each compound show its conjugate base. Lone pairs have been left out but are assumed to be t Show all valence electrons. Show any resonance structures if applicable. b) Rank the conjugate bases in the order you would predict, from most stable to least stable. c) Rank the original compounds in order, from strongest acid to weakest acid. OH Br OH OH OHarrow_forward
- Nonearrow_forwardAnswer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. A table of pka values for various organic and inorganic acids can be found in the references section. H2 H2 CH3 H2C=CH2 CH OH H2C=CH D A ethoxide B ethene ethanol ethene anion a) The weaker acid is b) Its conjugate base is c) The species that predominate at equilibrium are (two letters, e.g. AC)arrow_forward2. Draw the products using curved arrows for the following acid base reaction. Label its conjugated acid-base pair. I—Z Base + N H. Acid H H searrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY