
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Arrange in order of increasing acidity.
Answer is: (lowest acidity) B<C<A<D (highest acidity)
Please explain reasoning
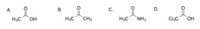
Transcribed Image Text:The image presents four different organic compounds, each with a letter label:
- **A.** Acetic acid: The structure is shown with a methyl group (CH₃) connected to a carboxylic acid group (COOH).
- **B.** Acetone: This compound is depicted with two methyl groups (CH₃) attached to a carbonyl group (C=O).
- **C.** Acetamide: Illustrated with a methyl group (CH₃) bonded to an amide group (CONH₂).
- **D.** Trichloroacetic acid: This structure features a trichloromethyl group (CCl₃) attached to a carboxylic acid group (COOH).
These illustrations provide a visual representation of the molecular structures, highlighting the specific functional groups and molecular frameworks associated with each compound.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the following compounds, put them in order from least to most acidic (based from most the acidic proton) hydrochloric acid, acetic acid, ethanol, water, ammonia, propyne, propene, cyclopentadiene, trichloroacetic acid, hydrogen sulfide phenol, 4-methoxyphenol, 4-nitrobenzoic acidarrow_forwardList the given compounds in order of increasing acidity (lowest first). Give a reason for your answer.arrow_forwardList the given compounds in order of increasing acidity (lowest first). Give a reason for youranswer.arrow_forward
- The following "thought questions" are based on our lecture on acidity and molecular structure. See if you can reason these out but don't spend too much time on them. 1. Choose the stronger acid in each pair and explain your reasoning. a) HNO2 and HNO3 b) H₂S and H₂O c) CCI3COOH and CH3COOH d) H₂SO4 and H₂SO4arrow_forwardboth questions goes together.... thanks!arrow_forward9. H. NH HH Which is a stronger acid? Explain your reasoning in a few sentences using concepts from the course.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY