
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
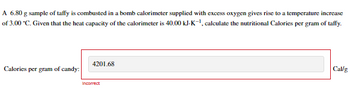
Transcribed Image Text:A 6.80 g sample of taffy is combusted in a bomb calorimeter supplied with excess oxygen gives rise to a temperature increase
of 3.00 *C. Given that the heat capacity of the calorimeter is 40.00 kJ-K-¹, calculate the nutritional Calories per gram of taffy.
Calories per gram of candy:
4201.68
Incorrect
Cal/g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
would this answer be following the correct number of significant figures
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
would this answer be following the correct number of significant figures
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer both the parts of question otherwise I'll downvotearrow_forwardFor 6.60 g of a substance initially at 14.5*C with a heat capacity of 0.400 J/g*C, what will be its final temperature if q = -51.9 Jarrow_forwardCalculate the energy required to heat 557.0 g of ethanol from 8.0 °C to 18.8 °C. Assume the specific heat capacity of ethanol under these conditions is -1 2.44 J-gK 1 Round your answer to 3 significant digits.arrow_forward
- please see attached imagearrow_forwardA researcher studying the nutritional value of a new candy places a 5.60 g sample of the candy inside a bomb calorimeter and combusts it in excess oxygen. The observed temperature increase is 2.41 °C. If the heat capacity of the calorimeter is 47.50 kJ.K-, how many nutritional Calories are there per gram of the candy? Calories per gram of candy: Cal/garrow_forwardHow much heat energy is required to raise the temperature of 0.361 kg of copper from 23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g ·°C). Express your answer with the appropriate units. • View Available Hint(s) HÀ ? heat = Value Unitsarrow_forward
- When 1.60 g of liquid PCI3 at 23.9 C is added to a solution of water at in a coffee cup calorimeter, the temperature of the solution increases to 43.8C. If the heat capacity of the solution and calorimeter is 421.7 J/C, calculate the molar heat of the PCI3. Answer to 0 decimal places in kJ/mol. Your Answer: Answerarrow_forward- 1 A sample of glass, which has a specific heat capacity of 0.670 J-g.°C¯', is put into a calorimeter (see sketch at right) that contains 150.0 g of water. The glass sample starts off at 94.1 °C and the temperature of the water starts off at 24.0 °C. When insulated container the temperature of the water stops changing it's 28.2 °C. The pressure remains constant at 1 atm. water dlo Calculate the mass of the glass sample. Be sure your answer is rounded to the correct number of significant digits. sample 18 Ar a calorimeter x10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY