
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
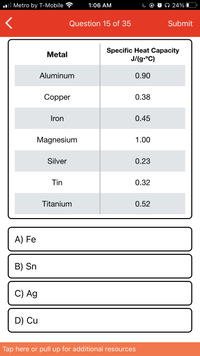
Transcribed Image Text:**Question 15 of 35**
**Select the metal with a specific heat capacity of 0.45 J/(g°C).**
| Metal | Specific Heat Capacity J/(g°C) |
|------------|--------------------------------|
| Aluminum | 0.90 |
| Copper | 0.38 |
| Iron | 0.45 |
| Magnesium | 1.00 |
| Silver | 0.23 |
| Tin | 0.32 |
| Titanium | 0.52 |
**Options:**
A) Fe
B) Sn
C) Ag
D) Cu
_Tap here or pull up for additional resources._

Transcribed Image Text:**Calorimetry Problem: Identifying a Metal by Its Specific Heat Capacity**
**Problem Statement:**
An 80.0 g sample of an unknown metal, initially at 96.0°C, is placed into 150.0 g of water at an initial temperature of 26.0°C in a calorimeter. After thermal equilibrium is reached, the final temperature of the water is 28.1°C. The objective is to determine the identity of the metal based on its specific heat capacity. The specific heat capacity of water is given as 4.18 J/g·°C.
**Data Table: Specific Heat Capacities of Various Metals**
| Metal | Specific Heat Capacity (J/g·°C) |
|------------|----------------------------------|
| Aluminum | 0.90 |
| Copper | 0.38 |
| Iron | 0.45 |
| Magnesium | 1.00 |
| Silver | 0.23 |
| Tin | 0.32 |
| Titanium | 0.52 |
**Task:**
Using the data provided and the principle of conservation of energy in calorimetry, calculate the specific heat capacity of the unknown metal. Compare the calculated value with the values in the table to identify the metal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the laboratory a student determines the specific heat of a metal.He heats 18.4 grams of platinum to 99.28 °C and then drops it into an insulated cup containing 85.3 grams of water at 23.56 °C. When thermal equilibrium is reached, he measures the final temperature to be 24.10 °C.Assuming that all of the heat is transferred to the water, he calculates the specific heat of platinum to be J/g°C.arrow_forwardA student is asked to identify an unknown piece of metal. The metal has a mass of 12.34 g. It is placed in a boiling water bath and brought up to 99.98 oC. A coffee-cup calorimeter is set up with 103.25 mL of water (density = 1.00 g/mL and specific heat = 4.184 J/g.oC) at a room temperature of 21.30 oC. The metal is removed from the boiling water and placed in the calorimeter. A final temperature is recorded as 22.32 oC. Find the specific heatcapacity of the unknown metal. (Assume there is no heat loss)arrow_forwardIn an experiment, 26.5 g of metal was heated to 98.0°C and then quickly transferred to 150.0 g of water in a calorimeter. The initial temperature of the water was 22.5°C, and the final temperature after the addition of the metal was 32.5°C. Assume the calorimeter behaves ideally and does not absorb or release heat.arrow_forward
- When 6.54 grams of Zn is placed in 500.0 mL of 1.00 M CuSO4(aq) in a coffee cup calorimeter, it reacts completely to displace copper. The temperature of the solution rises from 20.0˚C to 30.4˚C. Assume the coffee cup itself gains no heat and that the solution has the same density (1.00 g/mL) and specific heat (4.184 J/g˚C) as pure water. (a) How much heat does the solution gain during this reaction? (in J)arrow_forwardA 19.3 g sample of beryllium at 96.7°C is placed into 46.2 mL of water at 20.2°C in an insulated container. The temperature of the water at thermal equilibrium is 32.0°C. What is the specific heat of beryllium? Assume a density of 1.00 g/mL for water. (See this table.) J/(g·°C)arrow_forward3. A 74.5 g piece of metal at 86.0°C is placed in 133 g of water at 21.0°C contained in a calorimeter. The metal and water come to the same temperature at 24.2°C. How much heat (in J) did the metal give up to the water? (Assume the specific heat of water is 4.18 J/g·°C across the temperature range.) J What is the specific heat (in J/g·°C) of the metal? J/g·°C 4. A 0.528 g sample of KCl is added to 51.7 g of water in a calorimeter. If the temperature decreases by 1.07°C, what is the approximate amount of heat (in J) involved in the dissolution of the KCl, assuming the heat capacity of the resulting solution is 4.18 J/g°C? J Is the reaction exothermic or endothermic? exothermicendothermic 5. When 2.56 g of methane burns in oxygen, 128 kJ of heat is produced. What is the enthalpy of combustion (in kJ) per mole of methane under these conditions? kJ/mol methane 6. Joseph Priestly prepared oxygen in 1774 by heating red mercury(II) oxide with sunlight focused through a lens. How much…arrow_forward
- A 4.81 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72°C. After the salt has completely dissolved, the temperature of the solution is 28.54°C. If 3.12 × 10³ J of heat was gained by the solution, what is the total heat for the dissolution reaction of the 4.81 g of salt? How many moles of the unknown salt were used in the reaction ? _______ J?arrow_forwardA sample of aluminum, initially at 5.0 °C, was placed in 18.0 g of water, initially at 25.0 °C. The final temperature of the water and the metal was 21.0 °C. The specific heat of water is 4.184 J g-1 °c-1 and aluminum is 0.940 J g-1 °C-1. Ignore the heat capacity of the container. What is the mass (in g) of the aluminum?arrow_forwardWhen a 6.50 g sample of solid sodium hydroxide dissolves in 100.0 g of water in a coffee-cup calorimeter, the temperature of the water rises from 21.6 to 37.8oC. Was the chemical reaction (dissolving the solid) endothermic or exothermic? How do you know? Write a balanced chemical equation for this process. Determine how many joules of heat (q) were involved in changing the temperature of the water. If the heat that changed the temperature of the water was a result of the chemical reaction, determine the ΔH of the chemical reaction in kJ/mol of sodium hydroxide.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY