
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Working Backwards Parte Deux.
a.First, identify which
b. Based on your answers to A and B, what kind of reaction occurred here, SN1 or SN2?
c.Suggest reagents and solvent that could accomplish this reaction.
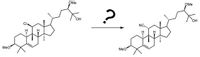
Transcribed Image Text:This image depicts a chemical transformation involving two steroid-like molecules.
**Left Molecule:**
- The structure features a steroid framework with a chlorine (Cl) atom attached, visible in the lower left segment of the molecule.
- Notably, there are methoxy (OMe) groups present on both the left and upper sides of the structure.
- A hydroxyl group (OH) is present at the top right, attached to an isopropyl group.
**Right Molecule:**
- Similar to the left molecule, this structure maintains the steroidal backbone.
- The chlorine (Cl) atom from the left molecule is absent and has been replaced with a nitrile group (–C≡N), indicating a substitution reaction.
- Methoxy (OMe) groups and the hydroxyl group (OH) remain in the same positions as in the left molecule.
The question mark with an arrow suggests an unknown or unspecified reaction or series of reactions leading from the initial compound on the left to the final product on the right. This type of diagram is common in organic chemistry to illustrate transformations without specifying reagents or conditions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Answer the following questions. a. Name the molecules shown below. Be sure to include stereochemistry (E/Z or cis/trans)! Br Br i. ii, HO b. What is the Markovnikov rule? Why is this rule followed in hydrohalogenation and hydration reactions? c. Draw the arrow-pushing mechanisms for the two reactions in parts c and d. Br-Br 'Br MeOH OMe d.arrow_forwardIn substitution reactions, (CH3)3C-I reacts at the same rate with Br and Cl even though Br is a more reactive nucleophile than Cl-. Why? Select one: A. (CH3)3C-Br reacts by SN1 mechanism whose rate is independent of nucleophile reactivity. B. The t-butyl group sterically hinders nucleophiles, making different nucleophiles appear to react at the same rate. C. (CH3)3C-Br reacts by SN2 mechanism and therefore all nucleophiles react at the same rate regardless of their reactivity. D. The t-butyl carbocation is so reactive, the measurable rate of it's reaction with different nucleophiles is imperceptible.arrow_forward2. Predict the major organic product(s) for each of the following reactions. Be sure to clearly indicate the proper stereochemical relationships between groups (if appropriate), and write the word "racemic" next to any compound that is formed as a racemic mixture. a. b. Ph CH3 CH3 C. ☑ OH d. NaOH H₂O + Мон H2SO4 OH CH2N2 Et2O CH3 DCC H3C OH Ph NH2arrow_forward
- 2-butanol (CH,CHOHCH,CH,) is classified as a(n). A. Choose the best reagents to complete the reaction shown below. C. primary alcohol secondary alcohol tertiary alcohol ether 10 What type of functionality does phenol contain besides the aromatic ring? B. D. Bean beeties can infest a pantry supply of dried beans. To control the beetles romone traps are used to attract the insects. Choose the best reagents to complete the following reaction in the synthesis of the dried bean beatle sex pheromone C amino alkene hydroxy ° alkyl E aldehyde с D 1.H B 20 A PB DMF E. Rank the compounds (shown below) in order of increasing acidity. на 20 F. P 500 pyridine PC DMF Br Choose the reagents that would be most likely to completion 818 A |<< B << C |<<\ D TTTTT D. Is an incorrect answer << |<<arrow_forwardIn an E2 reaction, what are the characteristics of a good base? How does a chemist tell a strong versus weak or bulky versus not bulky nucleophile? What is the best type of solvent for this reaction? Please define the terms.arrow_forward17. a. Label the reactive features, highlight the most reactive one, then highlight what it needs. Also, state if the reaction will start to create a carbocation, carbon radical, or carbanion, or will cause loss of aromatic character. If a carbocation, carbon radical, or carbanion starts to develop, label where that will occur. CH3 with Cl, and FeCl, CH2 CH3 b. Use mechanism arrows to illustrate the reaction that occurs. c. If applicable, use stabilization resources to deal with the carbocation, carbon radical, or carbanion that starts to develop during the reaction, and draw the structure of any resonance-stabilized intermediate. d. Continue labeling and diagramming the reaction until you find the major stable product(s). e. Finally, state the stereochemistry of the major product(s) and use either Fisher projection or perspective formula representations to illustrate that stereochemistry.arrow_forward
- Fill in the blanks in the following SN2 reactions with either the appropriate nucleophile,electrophile, or product and include any stereochemistry where it is relevant.arrow_forwardhelp; the reactions are all covered in Organic Chemistry, 6th Edition by Marc Loudon and Jim Parise. Chapter 15-17arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY