
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
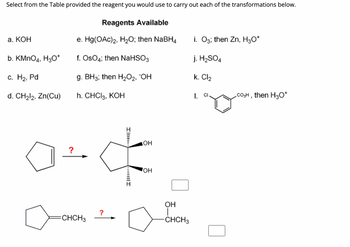
Transcribed Image Text:**Chemical Reaction Reagents and Transformations**
**Instructions:**
Select from the available reagents the one you would use to carry out each of the transformations below.
**Reagents Available:**
- a. KOH
- b. KMnO₄, H₃O⁺
- c. H₂, Pd
- d. CH₂I₂, Zn(Cu)
- e. Hg(OAc)₂, H₂O; then NaBH₄
- f. OsO₄; then NaHSO₃
- g. BH₃; then H₂O₂, ⁻OH
- h. CHCl₃, KOH
- i. O₃; then Zn, H₃O⁺
- j. H₂SO₄
- k. Cl₂
- l. Cl, CO₃H, then H₃O⁺
**Transformations and Reagents Selection:**
1. **Transformation 1:**
- **From:** Cyclopentene
- **To:** Cis-1,2-cyclopentanediol
- **Reagent to Use:** f. OsO₄; then NaHSO₃
- **Explanation:** Osmium tetroxide (OsO₄) followed by sodium bisulfite (NaHSO₃) is commonly used for dihydroxylation, converting an alkene to a vicinal diol with syn-stereochemistry.
2. **Transformation 2:**
- **From:** 1-Methylcyclopentene
- **To:** 2-Methylcyclopentanol
- **Reagent to Use:** g. BH₃; then H₂O₂, ⁻OH
- **Explanation:** Hydroboration-oxidation (BH₃ followed by H₂O₂ and hydroxide) leads to anti-Markovnikov hydration of alkenes, forming alcohols at the least substituted carbon of the double bond.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- destion 1 What product is expected from the reaction below? 1. Mg /dry ether 3. H*/H;0 МаBr OH 2. 3 A. 1 O B. 2 OD. 3 OE. 4arrow_forward3. Provide mechanisms for the following reactions. a. OH b. C. Н2 180 18OH cat. H 180 H₂O cat. H+ EtOH + CH3CH2CH2CO₂H NH cat. CH3SO3H HO C=N:arrow_forwardCan u clarify what optically pure meansarrow_forward
- What are the products of the given reactions?arrow_forward8. What is the order of increasing acidity for the following compounds? Br I CH3CH₂COOH BrCH₂CH₂COOH CH3CH₂CH₂OH CH3CHCOOH I IV, II, I, III I, II, IV, III c. III, I, II, IV d. III, IV, II, I e. I, II, III, IV a. b. II III IVarrow_forwardFind the products for these two reactions. Make sure to include relevant hydrogens, deuteriums and stereohemistry.arrow_forward
- Give the major organic product of the reaction.arrow_forwardComplete the following tree of reactions by providing their major products: HCI i. BH3 ii. NaOH, H₂O2, H₂O 03, (CH3)2S H+, H₂O Br₂ H₂, Pd/C CH₂CO3H Br₂, H₂O modellen H+, CH3OHarrow_forwardWhich of the following compounds produce the 3 products shown on reaction with KMnO4 and acid (H+). a. I b. II c. III d. II and IIIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY