
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
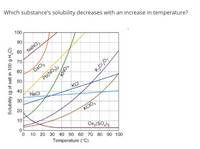
Transcribed Image Text:Which substance's solubility decreases with an increase in temperature?
100
90
NANO
80
70
60
CaCl,
50
Pb(NO,)2
40
KC
NaCl
30
20
KCIO,
10
Ce,(SO)3
10 20 30 40 50 60 70 80 90 100
Temperature (°C)
Solubility (g of salt in 100 g H,O)
SONY

Transcribed Image Text:NANO3
Ce2(SO4)3
KCI
KNO3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- (Please don't use hand raiting)arrow_forwardhow many atoms of each element is in the molecule 4SrO^-2arrow_forwardA Callister Solution MX O file:///C:/Users/Stephen%20Amoah%20Antwi/Desktop/Callister%205olution%20Manual_1250pp.pdf Find on page electronic materials た No results Options 1074 of 1250 E Contents 十9 O Fit to page CD Page view A Read aloud 1 Add notes 18.26 (a) In your own words, explain how donor impurities in semiconductors give rise to free electrons in numbers in excesS of those generated by valence band-conduction band excitations. (b) Also explain how acceptor impurities give rise to holes in numbers in excess of those generated by valence band conduction band excitations. 9:44 AM O Type here to search 2/20/2021 hp MDarrow_forward
- 6.a)What do covalently bonded atoms form? __________________________ b) Describe the attractive forces between the atoms in a molecule and between molecules. Why do molecular compounds have low melting and boiling points compared to ionic compounds? Covalent compounds are soft and squishy compared to ionic compounds. Describe the analogy used to help us understand this comparison. Do covalent compounds conduct electricity when they are mixed with water? Explain.arrow_forwardAtoms become stable when their outer shell is and has valence electrons. O full..10 O full..8 O half full...8 O half full..4 4arrow_forward50 grams of KCl are dissolved in 200 grams of water at room temperature. Is the resulting solution unsaturated, saturated, or supersaturated. Use the graph belowarrow_forward
- write the balanced chemical equation for P+O2=P2O5arrow_forwardThe adjacent line in the pere ratational spectrum of 35 CI 19F are separated by a frequency of 1.13×1." Hz, what is the inter atomic distance of this molecule ?arrow_forwardA material that conducts electricity but not as well as a metal but better than a non metal isarrow_forward
- If an electron is removed from each molecule, it is observed that N2+ has a weaker bond than N2, but O2+ has a stronger bond than O2. Explain why electron removal has a different effect on these two molecules.arrow_forwardHow culculat the founer tran form? f(t) = 1+ Cos (Wot).arrow_forwardQuestion helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON