
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
in this practice problem please break down each reagent and what it would do to the molecule and show the right answer, but show the product of each reaction 1, 2 , and 3
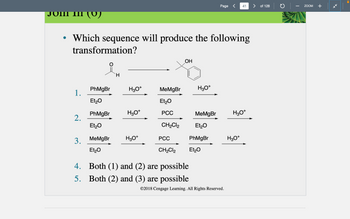
Transcribed Image Text:1.
• Which sequence will produce the following
transformation?
2.
3.
PhMgBr
Et₂O
PhMgBr
Et₂O
MeMgBr
Et₂O
H
H3O+
H3O+
H3O+
MeMgBr
Et₂O
PCC
CH₂Cl2
OH
H3O+
4. Both (1) and (2) are possible
5.
Both (2) and (3) are possible
MeMgBr
Et₂O
PhMgBr
PCC
CH₂Cl2 Et₂O
Page <
©2018 Cengage Learning. All Rights Reserved.
41
H3O+
H3O+
> of 128
ZOOM
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine which mechanism below is the most correct. Be sure to look at whether the most acidic H is reacting, if the direction of the arrows are correct, and if the correct products are formed based on the mechanism. If the correct answer is Option B, then explain why the other options are incorrect? Explain each option that is incorrect individually.arrow_forwardThe major product of this reaction exists as two stereoisomers. Draw both isomers. Show all hydrogen atoms in the structures. Use wedge and dash bonds to indicate the stereochemistry. H XE CH3 (large H3C H3C-CH₂ Br-Br H₂O excess) You can quickly add condensed hydrogens by clicking the More button and using the +H button. Select Draw Rings More Erasearrow_forwardSolve all parts otherwise I will downvotearrow_forward
- Just need to check answer, dont need lengthy explanation.arrow_forwardOffice Frame 4Bi + 302 → 2B1¿O3 Build your Model: Element Color Key # of Atoms # of Atoms (Reactants) (Products)arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts.arrow_forward
- Predict the major new product resulting from each of the following. Please do them step by step. Just dont use the sample form cause i dont understand those stick form so confusing...arrow_forwardChoose any EAS reaction. For your choice show an arrow-pushing mechanism using the compound below as the reactant. Include all resonance structures of intermediates where applicable. Consider whether the compound above favors ortho, meta and/or para additions, explain why it favors which of those it does. Be brief and to the point.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY