
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
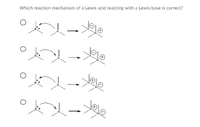
Transcribed Image Text:Which reaction mechanism of a Lewis acid reacting with a Lewis base is correct?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . Give an example of a Lewis acid-base pair and a frustrated Lewis acid-base pair. What is the difference between these two systems? What is the mechanism for the reaction with H2?arrow_forwardSince all of these questions are connected I'm asking them at once, thanks The reason that ammonium chloride has so many potential uses is because the ammonium ion is weakly acidic. Write the ionization reaction of the ammonium ion with water. This reaction happens in equilibrium. Based on the information you have, what can you say about the value of the equilibrium constant for the reaction in #9? Explain your reasoning. 3. Let’s say you have a solution of ammonium at equilibrium (meaning it is abiding by the reaction you wrote for #9). If you add more water, how will that affect the reaction? Explain your reasoning.arrow_forwardFor the following reaction, the products are favored at equilibrium. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases. ? C17H1903N + HF C₁7H1903NH* + F* C17H1903N F™ HF C17H1903NH+ Clear All Stronger Bronsted- Lowry acid Weaker Bronsted-Lowry acid Stronger Bronsted- Lowry base Weaker Bronsted-Lowry base Previousarrow_forward
- How Lewis acid–base reactions illustrate a general pattern of reactivity ?arrow_forwardThese two nitrogen atoms are potentially basic. Write the two products of the these N atoms with BF3. Which of the two N atoms will be more basic? Justifyarrow_forwardPlease don't provide handwriting solutionarrow_forward
- Which of the following base would you use to achieve the following reaction? Br A) KOET В кон t-BUOK D) NH3arrow_forwardWrite the acid dissociation constant expression (Ka ) for the dissociation of HCN.arrow_forwardAny reaction in which one species donates an electron pair to another species is a Lewis acid–base reaction. Explain this ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY