
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
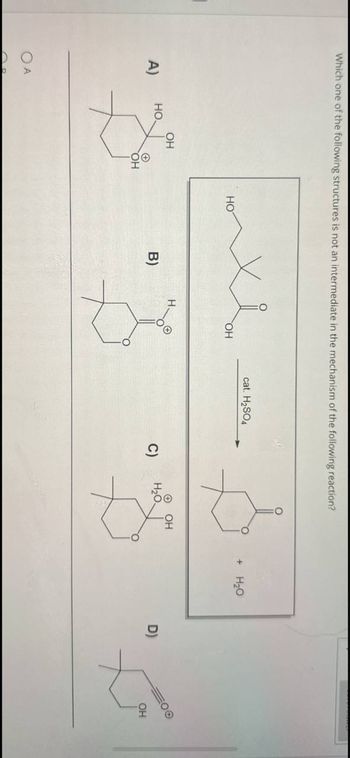
Transcribed Image Text:Which one of the following structures is not an intermediate in the mechanism of the following reaction?
A)
OA
HO
OH
HO
хе
cat. H₂SO4
+ H₂O
OH
OH
B)
་་
C) H₂O.
OH
D)
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Mechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 wand woled mot ozsm E Step 3 N-H C-OH C=N-H Step2 C=N-H H-O-H H₂O motno vgiene fesrigid onlt to smotnos -C=N-H a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. H₂O: rate determining step to noipojovo hamwell sit 5(emise erit voittons 10) vanas mi sdgin al rainW di of E^ reaction progress →arrow_forwardChemistry please answer in tipping formatarrow_forwardWhich series of reactions would carry out the following transformation? OTS ? ► View Available Hint(s) NH₂ O 1. NH3 2. NaBH4 O 1. Phthalimide, KOH, H₂O 2. NH₂NH₂, heat O 1. NaN3 2. LIAIH4 3. H₂O O 1. NaCN 2. H₂, Pdarrow_forward
- The compound shown here can be synthesized from what starting compound? ОН A) 1-hexanol B) 2-hexanol C) cyclohexene D) cyclopentene E) pentene +arrow_forwardPlease help me with both of these problems, they are the same question its just a two-part, I really want to study so please helparrow_forwardWhich sequence of reactions will complete the transformation below? 1) BH3-THF, 2) NaOH, HOOH, 3) NaOEt 1) HBr, 2) ethanol, heat 1) BH3-THF, 2) HOOH, NaOH, 3) NaH (strong base), 4) ethyl iodide 1) HBr, ROOR, heat, 2) NaOEt OHBr, ethanol, heat LOCH₂CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY