
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
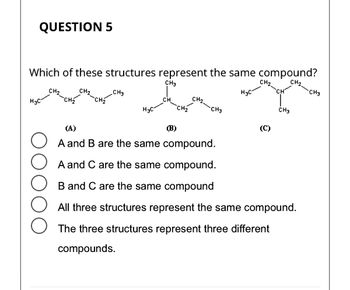
Transcribed Image Text:**Question 5**
**Which of these structures represent the same compound?**
- Structure (A): CH₃-CH₂-CH₂-CH₂-CH₃
- Structure (B): (CH₃)₂CH-CH₂-CH₃
- Structure (C): CH₃-CH(CH₃)-CH₂-CH₃
Options:
- ☐ A and B are the same compound.
- ☐ A and C are the same compound.
- ☐ B and C are the same compound.
- ☐ All three structures represent the same compound.
- ☐ The three structures represent three different compounds.
Expert Solution
arrow_forward
Step 1
Isomers are the species having same moleculer formula but different structures ,i.e. atoms or groups attached in different way.
The compound (A) ,(B) and (C) have same molecular formula(C6H14) but the structures are different . Hence these are constitutional isomers.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following molecule. Remember to use dashes, commas and spaces as appropriate, to alphabet your substituents, and to spell correctly! CH H2 C. CH H2 CH H,C H2 H2arrow_forwardGive the systematic name of each of the compounds shown in the picture.arrow_forwardName the following groups. CH3- CH3CH₂- CH3 CH3C- CH3 group group grouparrow_forward
- Which of following structures represents the structural formula for methane, CH? H H-C- Н Н H-C-H H И Н он-с-с-н Он Н C- C H H Нarrow_forwardGive the common name for 0 || CH3 C−N–CH,–CH,—CH3 Spell out the common name of the compound. Part F CH3 Submit Request Answer Give the IUPAC name for O CH₂ CH3 C-N–CH–CH3 Spell out the IUPAC name of the compound.arrow_forwardA model of a cycloalkane appears in the window below. ball & stick labels Which of the following represent structural isomers of the molecule shown in the model? Choose all that apply. ҫHз CH2 CHз Cн -CH2 Cн, CCH-CHz-CH3 CH CH3 CH3-CH-CH2-CH сHз CH -CH сн -CH2 CH3 сHз CH CH2arrow_forward
- Which of these structures represents an improper structure (one in which one or more atoms does not have the normal number of bonds)? CH₂ CH₂ CH,CH,CH,CH, CHy (1) H₂C 000000 CH₂ CH₂ (1) (2) (3) (4) (5) (6) CH₂ CH₂ (4) CH3 H₂C CH₂ (2) CH₂CHCH₂CH₂ CH₂ (5) (CH3C CH₂CH(CH3)2 (3) (CH3CH₂CH₂CH3)2 (6)arrow_forwardProvide the following: 1. expanded structural formula 2. Molecular formula " CH3 CH2 | CH2 — CH2 — СH — CHF — CH2 — СНЗ CH2 CH3arrow_forwardDraw the skeletal structures for these two molecules:arrow_forward
- Analyze the two Newman projections and determine the relationship between the two. How would you describe the relation between conformations when they are maintained at a temperature too low to permit them to interconvert? CH3 CH3 Br. H H H H A. Identify the relationship. They are identical. They are structural isomers. They are stereoisomers. They are conformers. H H -I H Br H B. What is the relationship at low temperatures? They are identical. They are conformational diastereomers. They are structural isomers. They are conformational enantiomers.arrow_forward1. Use a Newman projection about the indicated bond to draw the most stable conformer for each compound. (a) 3-methylpentane about the C2 ¬ C3 bond (b) 3,3-dimethylhexane about the C3 ¬ C4 bond2. refer to the questions below:arrow_forwardGive the systematic name of each of the compounds shown in the picture.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY