
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
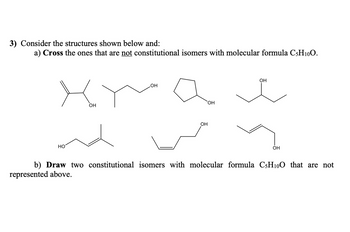
Transcribed Image Text:3) Consider the structures shown below and:
a) Cross the ones that are not constitutional isomers with molecular formula C5H10O.
HO
OH
OH
OH
OH
OH
OH
b) Draw two constitutional isomers with molecular formula C5H10O that are not
represented above.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 2) a) Provide Newman projects of the three staggered conformations of molecule 1 shown below looking down the indicated bond (1→2). Circle the most stable conformation. HH3C CH3 H molecule 1 b) Provide Newman projects of the three eclipsed conformations of molecule 1 looking down the indicated bond. Put a square around the least stable conformation.arrow_forward7) How many isomers can you construct for the molecular formula C,H,O? Use a red ball with two holes for the oxygen atom. Draw a dash structural representation of each structure.arrow_forwardDraw at least four isomers with molecular formula given below. C4H10Oarrow_forward
- The structures below are H. H CH₂ H H CH₂ A) not isomers B) conformational isomers C) cis-trans isomers D) structural isomers E) both B and D H H H CH3 H CH3arrow_forwardFrom the molecule C7H13N, draw (IF YOU CAN): a)constitutional isomer that has one quarternary carbon b) constitutional isomer that has one tertiary carbon c) constitutional isomer that has only primary and secondary carbons d) constitutional isomer that has one asymmetric carbonarrow_forwardWhich of the following pairs of compounds are cis-trans isomers? O HC = C- CH3 and CH3 - C = CH O CH3, CH3 O CH3. CH3 O CH3, H O CH3, H C=C C=C C=C C=C Com CH3 CH3 H H H H CH3 H DESA and and and and H H H H H H c=c C=C₁ c=c CH₂ H CH3 CH₂CH3 CH3 H CH3 CH3 C=C H CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY