
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
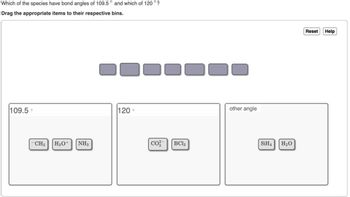
Transcribed Image Text:Which of the species have bond angles of 109.5° and which of 120° ?
Drag the appropriate items to their respective bins.
109.5°
CH3 H3O+
NH3
120°
co
BCl
other angle
SiH4 H₂O
Reset
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- What typically is found in position X in the molecule below?arrow_forwardDesign a cation exchange experiment to purify a protein with a pl of 4.2. Charge of the beads on the column: Correct answer: Negative pH of the buffer: Incorrect answer: 4.5 Counter-ion used (Na or Cl): Correct answer: Na +arrow_forwardSpecify whether each condition would result in increasing or decreasing acidity by dragging each label into the appropriate box. Labels Drop Zones Reset All Adding 1M Ht to a Increases Acidity (1/2) Decreases Acidity solution (2/2) Adding 1M H to a solution while simultaneously adding 2M (-OH) Removing H* Decreasing pH Adding base Adding 1M (OH) to a solution Removing acidarrow_forward
- Based on the given figure, answer the following questions H CH₂OH H OH H O H OH H J H Glucose CH₂OH H OH alpha 1, 6 H H beta 1, 4 alpha 1, 4 O H CH₂OH H OH H OH H OH O H H J 1. o CH₂₂ H OH H H O The monomer of this molecule is? What is the branching point? OH H H J The name of this polymer in plants? The importance of this molecule o The main glycosidic bond in the chain? Sucrose Fructose Galactose CH₂OH H OH O H OH Structural sugar H Harrow_forwardpls send me answer of this question with explanation in typing form only ,so i will rate you sure sir and also show electronic configuaration.arrow_forward12 A Aa v AaBbCcDc AaBbCcDdEe AaBbCcDdE AaBbCcDdE Uv ab x, x A A v 三三三=|這、| 4、 Caption Emphasis Heading 1 Heading 3 Q2. Complete the table below with a tick to show which of the following statements describe the molecules. Statement Alpha glucose Fatty acid Amino Nucleotide acid A hexose sugar Insoluble in water Formed by condensation reactions Contain the elements, C, H and Contains the element, N Has a carboxyl group, -COOH Forms polypeptides >arrow_forward
- Given the active site diagram below, identify the acidic residue from the indicated components. 2 1 4 3 5 LO 5 2 S 4 -3arrow_forwardWhat is the charge of GGFEKILMP at pH=7.5 O +2 0 O +1 O-1 O -2arrow_forwardDraw the Lewis structures and determine which of these molecules has a central atom that unavoidably violates the octet rule. XeF2 OSO2 OPCI3 CS₂ OBCI 3 NO3 000 O SO3 IFS NH3 xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON