
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
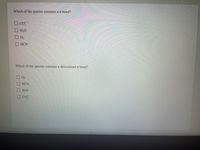
Transcribed Image Text:Which of the species contains an bond?
O co;-
O H2O
O 03
HCN
Which of the species contains a delocalized a bond?
O 03
HCN
H20
O O O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Formula Lewis Electron Molecular Bond Polar or Attractive Structure Pair Geometry Angle Force Non Polar Geometry Between Molecules CH4 Around First C Around First C C2H4 Around First C Around First C C,H2 Around First C Around First C CH;OH Around O Around O C2H;OH Around O Around O CH20 CH,OCH, Around O Around O CH;COOH Central C Central C НСООН Central C Central C CH;NH2 Around N Around N CH;COCH; Central C Central Carrow_forwardRank in order of increasing strength of the indicated bond. (1 = weakest bond; 4 = strongest bond) A B A 1. V H B H H C D Harrow_forwardClO- ,does it have linear (180o bond angle) or tetrahedral (o ?) VSEPR shape.arrow_forward
- Which is more polar, H-Br or H-O?arrow_forwardEstimate ΔrH° for forming 2 mol ammonia from molecular nitrogen and molecular hydrogen. Is this reaction exothermic or endothermic?arrow_forwardThe bond energy of a C—C single bond averages 347 kJ mol-1; that of a CC triple bond averages 839 kJ mol-1. Explain why the triple bond is not three times as strong as a single bond.arrow_forward
- Explain how the dipole moment could be used to distinguish between the cis and trans isomers of 1,2-dibromoethene:arrow_forwardDraw in the orbitals for the oxygen's lone pairswhich structure allows the oxygen lone pair to be in an orbital that is aligned with the pi bond?Draw in the p orbitalsCircle the atoms in each structure that are conjugatedarrow_forwardDetermine the number of o bonds and bonds in each of the molecules. H₂C=CC1₂ number of o bonds: HOOC–COOH number of o bonds: FHC=C=CHF number of o bonds: H H x10 number of o bonds: H H TOOLS number of bonds: number of bonds: number of bonds: number of bonds:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning