
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
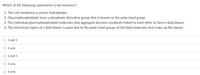
Transcribed Image Text:Which of the following statements is/are incorrect?
1. The cell membrane is purely hydrophobic.
2. Glycerophospholipids have a phosphate derivative group that is known as the polar head group.
3. The individual glycerophospholipid molecules that aggregate become covalently linked to each other to form a lipid bilayer.
4. The interfacial region of a lipid bilayer is polar due to the polar head groups of the lipid molecules that make up the bilayer.
O 1 and 3
O 1 only
O 2 and 4
O 3 only
O 4 only
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 1. What characteristics of phospholipid molecules cause them to form a membrane with two layers? Sketch a diagram to help you explain.arrow_forwardWrite 2 (any) functions of membrane carbohydrates and explain the functions.arrow_forwardWhich statement is correct about the phospholipids in membranes? The polar heads face inward The non-polar tails face outward The non-polar tails are hydrophilic A bilayer forms with the heads facing outward and the tails facing inwardarrow_forward
- Which of the following molecules may diffuse freely through a phospholipid bilayer? (select two answers) asparagine, an amino acid with a large, hydrophilic side chain alanine, an amino acid with a small, hydrophobic side-chain testosterone, a steroid hormone fructose, a monosaccharide molecular oxygen (O2)arrow_forward1) Placing phospholipids in water will spontaneously form a spherical structure called a:Group of answer choices lumen ball sphingoid cytoskeleton liposome 2) Saturated fatty acidsGroup of answer choices Have each carbon "saturated" with hydrogen Have each carbon "saturated" with other carbons Have each carbon "saturated" with hydrogen and are solids at room temperature Are solids at room temperature Contain double bonds 3) In intestinal epithelia, glucose can be taken into cells against its concentration gradient. How can glucose be transported against its concentration gradient without the direct expenditure of ATP in intestinal epithelial cells?Group of answer choices It uses simple diffusion It uses the Glut1 transporter It uses the Glucose ATPase It uses the Glucose/Na+ symporter None of the above. Due to the Second Law of Thermodynamics, molecules cannot be moved against their concentration gradient.arrow_forwardChoose the best answer 1) If the lipid bilayer in Section B represents the plasma membrane, which part of the protein is the cytoplasmic domain? There is no way to tell which is the cytoplasmic domain with the information given. Section A is the cytoplasmic domain since it contains disulfide bonds. Section C is the cytoplasmic domain since the N-terminus is always found in the cytoplasm. Section C is the cytoplasmic domain since glycosylation cannot occur on the cytoplasmic domain. Section B is the cytoplasmic domain since it is an alpha-helix. 2) Which type of amino acids is most commonly found in Section B? Hydrophilic Hydrophobic There is no way of knowing which type of amino acids is most commonly found in Section B with the given information Charged 3) If the lipid bilayer in Section B represents the ER membrane, which part of the protein is found INSIDE the ER (in the ER lumen)? The entire protein must be inside the lumen of the ER. Section C is inside the ER…arrow_forward
- Consider the following diagram of the cell membrane. Which of the statements is NOT true regarding the cell membrane? Group of answer choices a. Proteins and carbohydrates are also components of the cell membrane. b. Certain phospholipids and proteins on the outer surface of the cell membrane are attached to carbohydrates. c. Some carbohydrates extend through the entire lipid bilayer and appear on both surfaces of the membrane d. Some of the proteins embedded in the cell membrane constitute channels (passage canals).arrow_forwardIf you place phospholipids in an oil-based solution, would they still self-assemble into a bilayer? If so, describe its structure.arrow_forwardIt seems paradoxical that a lipid bilayer can be fluid yet asymmetrical. Explain.arrow_forward
- Which of the following statements are TRUE about the phospholipid bilayer? Select all that apply. Integral proteins have at least one part touching the inner 'tails' of the membrane. Peripheral proteins span the entire membrane. Cholesterol can be used to make the membrane more OR less fluid, depending on the temperature. Glycolipids and glycoproteins face the inside of the cell.arrow_forwardLipid bilayers form spontaneously in a process driven by the hydrophobic effect. Explain how the hydrophobic effect drives bilayer formation from individual lipids in an aqueous environment. Describe how the physical properties of the lipid bilayer are determined by the chemical properties of the membrane lipid components. Diagrams are encouraged for both parts of the question.arrow_forwardWhich molecule would you predict moves through a lipid bilayer most rapidly without the help of a transport protein? Group of answer choices A NH2SO2CH3 B C6H12O6 C C2H4 D CH3OH E C2H5OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON