
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
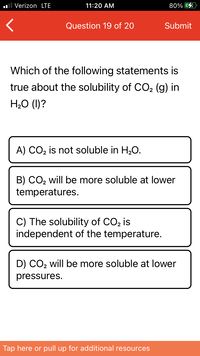
Which of the following statements is true about the solubility of CO2 (g) in H2O (l)?
Transcribed Image Text:Verizon LTE
11:20 AM
80%
Question 19 of 20
Submit
Which of the following statements is
true about the solubility of CO, (g) in
H2O (1)?
A) CO, is not soluble in H,O.
B) CO2 will be more soluble at lower
temperatures.
C) The solubility of CO, is
independent of the temperature.
D) CO2 will be more soluble at lower
pressures.
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. An effluent waste stream contains 3300mg/L organic matter ((CH2O) suspended and dissolved) and 27mg / L ammonium ion NH4+ (as N). Write chemical equations for the complete oxidation of (CH2O) and NH4+ and calculate the total BOD of this effluent in mg/L O2arrow_forwardI had 1.0 mL of 1.0M CoCl2 in a test tube. I put it into boiling water. [Co(H2O)6]2+, [CoCl4]2- Equilibrium temperature effect. Effect of heat What happens to the equilibrium? Use this equation in your explanation... 4Cl_ (aq) + [Co(H20)6]2+(aq)---[CoCl4]2-(aq)+ 6 H20 (l) I did this in class. The color change was very slow but it changed from a burgandy to fushia.arrow_forward3.02 g of sodium sulfate was mixed into a solution containing 48.5 mL of 0.185 mol/L aqueous silver nitrate: Calculate the maximum mass (in g) of precipitate that can form. Note: Balanced chemical eq. with state of each compound required. 2. What is the molar concentration of sodium ions in the resulting solution?arrow_forward
- 16.arrow_forwardIn which of the following solutions would PbBr₂ be the most soluble? O 0.10 M Pb(NO3)2 O 0.20 M KNO3 0 0.15 M NaBrarrow_forwardWhat effect will adding CO2(g) have on this: CO2(g) + C(solid graphite) <=> 2 CO(g). The equilibrium constant will increase. The reaction will shift to the right in the direction of products. The reaction will shift to the left in the direction of reactants.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY