
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can’t decide if the answer is a or c? Help please
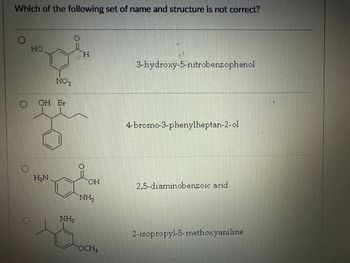
Transcribed Image Text:Which of the following set of name and structure is not correct?
HO
H
NO2
OH Br
3-hydroxy-5-nitrobenzophenol
4-bromo-3-phenylheptan-2-ol
H₂N
OH
2,5-diaminobenzoic acid
NH2
NH2
OCH3
2-isopropyl-5-methoxyaniline
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 18-28 Arrange these compounds in order of increasing acidity: benzoic acid, benzyl alcohol, phenol.arrow_forward14. What is the IUPAC name for the following compound? NH- (a) propyl propanamide (b) propyl propanoate (c) N-propylptopanamine. (d) N, N-propylpropanamide (e) N-propxlpropanamidearrow_forwardIdentify the conjugate acid/conjugate base pairs for the structures below.arrow_forward
- LSD (a hallucinogen) and codeine (a narcotic) are structurally more complex derivatives of 2-phenylethanamine. Identify the atoms of 2-phenylethanamine in each of the following compounds.arrow_forward2-chloropentanoic acid; 2-fluoropentanoic acid pentanoic acid 2-methylpentanoic acid Rank in acidityarrow_forwardPentanoic acid is More or Less soluble in water than pentanol Pentanoic acid is More or Less soluble in water than octanoic acid Pentanoic acid is More or LesS soluble in water than pentanedioic acid Acidic, Basic or Amines are (Select Neither Amides are (SelecAcidic, Basic, or Neither Acidic, Basic, or Carboxylic acids are 19 neither More or Lessar than the o-H bond The N- H bond is (Se Higher or Lower boiling point than pentanol. Pentanamine has aarrow_forward
- Give clear detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardMay you please help me with this ochem question?arrow_forward1 2 3 4 Draw The Structures? Z-Butenedioic acid (maleic acid) 2,5-Dimethylbenzoic acid Z-2-Chloro-3-phenyl-2- propenoic acid (cis-B- chloroallocinnamic acid) Decanedioic acid (sebacic acid) E-3-Phenyl-2-propenoic acid (trans-cinnamic acid)arrow_forward
- which two of the following will react with weak base; sodium bicarbonate and which two will react with strong base; NaOH and which two will remain neutral. Write chemical reactions as well. COOH benzoic acid MP 122°C COOH o-toluic acid MP 101°C g 2-naphthol MP 123°C OH OH C(CH3)3 p-tert-butylphenol MP 101°C денз OCH3 p-dimethoxybenzene MP 57°C naphthalene MP 80°Carrow_forwardCan you please give an explanation for question 6 ( continues to 2nd page)? Thank youarrow_forwardIn the mid-1930s a substance was isolated from a fungus that is a parasite of ryes and other grasses. This alkaloid, lysergic acid, has been of great interest to chemists because of its strange, dramatic action on the human mind. Many derivatives of lysergic acid are known, some with medicinal applications. Perhaps the best known derivative of lysergic acid is the potent hallucinogen lysergic acid diethylamide (LSD): మగవా జి N-H LSD (CH25N;O) Like other alkaloids, LSD is a weak base, with Kp = 7.6 × 107. What is the pH of a 0.94 M solution of LSD? pH =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning