
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
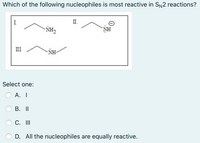
Transcribed Image Text:**Question:**
Which of the following nucleophiles is most reactive in \( S_N2 \) reactions?
**Structures:**
- **I.** \( \text{C}_2\text{H}_5\text{NH}_2 \)
- **II.** \( \text{C}_2\text{H}_5\text{NH}^- \)
- **III.** \( \text{C}_6\text{H}_5\text{NH}_2 \)
**Options:**
- A. I
- B. II
- C. III
- D. All the nucleophiles are equally reactive.

Transcribed Image Text:**Question:**
Examine the alkyl bromides below. Which is the most reactive as a substrate in \(S_N2\) reactions with \( I^- \) (acetone solvent)?
**Structures:**
- **Structure I**: Tertiary butyl bromide (2-bromo-2-methylpropane)
- **Structure II**: 1-bromobutane
- **Structure III**: 1-bromopropane
- **Structure IV**: Methyl bromide
**Select one:**
- A. IV
- B. I
- C. II
- D. III
- E. All are equally reactive
**Description of the Structures:**
1. **Structure I** is a tertiary alkyl bromide with the bromine atom bonded to a carbon that is connected to three other carbon atoms.
2. **Structure II** is a primary alkyl bromide where the bromine is bonded to a carbon connected to one other carbon atom and two hydrogen atoms.
3. **Structure III** is similar to Structure II but with a shorter chain.
4. **Structure IV** is a methyl bromide with the bromine atom bonded directly to a methyl group (CH\(_3\)).
**Explanation:**
In \(S_N2\) reactions, the reactivity is highest for primary halides and lowest for tertiary halides. This makes Structure IV (methyl bromide) the most reactive option due to its minimal steric hindrance, allowing for faster nucleophilic attack.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which one of these provides the strongest nucleophile in an SN2 reaction? a) HC=N b) NaC-N c) NH3 d) NH4 O b P. C. a.arrow_forwardWhich of these is the rate-determining step in the nitration of benzene? A. Protonation of HNO3 B. Loss of H2O by HNO3 C. Formation of sigma complex D. Loss of H+ by sigma complex E. Protonation of H2SO4arrow_forwardWhich of the following statements correctly describe(s) SN1 reactions of alkyl halides (RX)? I. Rate = k[nucleophile] III. Rate=k[RX] [nucleophile] II. Rate = K[RX] IV. The reactions occur in two or more distinct steps. V. Rearrangements are sometimes seen. B) II and V C) I, III, and IV A) III and IV D) I only E) III, IV, and V F) III onlyarrow_forward
- What would be the major product of the following reaction sequence? 1. HBr, ROOR, hv 2. BuOK, t-BuOH, heat Of-Bu p. O I Select O O one: a. I b. ll c. Ill d. IV e. V II III IV Ot-Bu Varrow_forwardSelect all of the molecules that will readily undergo a 1,2-hydride or a 1,2-methyl shift to form a more stable carbocation.arrow_forward1 1 Provide a reasonable mechanism to account for the following reaction. NO2 H,SO4 HNO; Propose a mechanism to account for the formation of TNT from toluene. Why is the reaction below a very difficult reaction of accomplish? NO2 NO2 x.s. HNO, H,SO, NO2arrow_forward
- Examine the above reactions (RXN 1-6). Choose the letter of your answer from the choices provided. RXN 1 is completed by RXN 2 is completed by RXN 3 is completed by RXN 4 is completed by RXN 5 is completed by RXN 6 is completed byarrow_forwardhomas Ral x Q For the reaction se XC For each of the re x QFor each of the re X Fo.edu/ultra/courses/_623768_1/dl/outline Question Completion Status: QUESTION 7 The reaction below proceeds via an E2 elimination. Using Newman projections explain the product distribution Br Attach File Browse Local Files QUESTION 8 KOtBu C For each of the re X Browse Content Collection 51% C For each of the re X A 18%arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY