
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
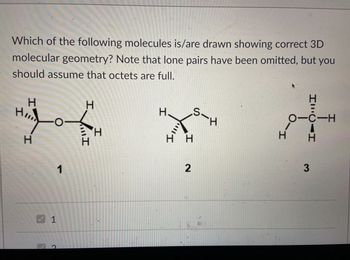
Transcribed Image Text:Which of the following molecules is/are drawn showing correct 3D
molecular geometry? Note that lone pairs have been omitted, but you
should assume that octets are full.
H
H
H
H
H
1
S-H
_H
H
H H
2
1
2
HILCAH
OTC-H
エーロ
H
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Formamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardIn each of the following molecules, a central atom is surrounded by a total of three atoms or unshared electron pairs: SnCl2, BCl3, SO2. In which of these molecules would you expect the bond angle to be less than 120? Explain your reasoning.arrow_forwardIndicate which of the following molecules are polar. Draw the molecular structure of each polar molecule, including the arrows that indicate the bond dipoles and the molecular dipole moment. (a) HCN (b) I2 (c) NOarrow_forward
- Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, TF5, and SCl6. These 12 compounds are all examples of different molecular structures. Draw the Lewis structures for each and predict the molecular structures. Predict the bond angles and the polarity of each. (A polar molecule has a net dipole moment, while a nonpolar molecule does not.) See Exercises 25 and 26 for the molecular structures based on the trigonal bipyramid and the octahedral geometries.arrow_forwardChloromethane has the Lewis structure _______________________________ The carbon atom is sharing 4 electron pairs. In each shared pair the carbon atom “owns” 1 electron. The number of electrons that “belong” to carbon is ___. Carbon, being a Group ___ element would have 4 , outer shell electrons in the unbonded, neutral state. Therefore, the carbon atom in chloromethane has a formal charge of zero.arrow_forwardThis compound has the formula C6H5CH3ClCH=CHCHO Each intersection of adjoining lines is a carbon atom. =C- or -C- a sketch of this molecule showing every hydrogen atom needed to complete octets and any lone pairs of electrons that are needed to complete the oxygen and chlorine atom octets Write by the following atoms the VSEPR geometry on the drawing as linear, bent, trigonal planar, bent, trigonal pyramidal or tetrahedral for the atoms: The carbon on the right hand side directly bonded to Oxygen The carbon that is CH3 on the left side The carbons in the ring (all the same)arrow_forward
- tR-XZxj_1SoekWMaP1As1fEhnC179H4SICzl1mdWKpKlvbF3amiKazP. OF ORGANIC MOLECULES Drawing a Lewis structure for a simple organic molecule from a. GE OOD D Draw a Lewis structure of the molecule that matches the description below. All non-H atoms should have full octets, and all formal charges should be zero. Unless you're told otherwise, assume there are no rings in the molecule. Description: The molecule is composed of 10, 4H's, and 2C's and it contains a C-C single bond. Click and drag to start drawing a structure. 2021 McGraw-Hill Education All Rights Reseved Terms of Uhe Pcy Check Explanationarrow_forwardPredicting the arrangement of electron groups around the central atom of a molecule.arrow_forward) Bean hexene is an odor compound used in cosmetics and cleaners. It is also known as leguminal. Its formula is: CH3CH2CHCHCH2CH2OCHCH2CH3 | OCH3 Please draw a Lewis structure for this compound with CORRECT GEOMETRY (use dashes and wedges!) Label all pi bonds in the molecule (if there are any) Indicate the bond angles for the red highlighted section of the molecule. (hint: you may want to keep the main carbon chain in-plane with the page for clarity) For this question, you don’t need to draw out the orbitals.arrow_forward
- ||| = 43°F Clear O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Predicting the arrangement of electron groups around the centr... Answer the questions in the table below about the shape of the chlorine pentafluoride (CIF) molecule. How many electron groups are around the central chlorine atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central chlorine atom? (You may need to use the scrollbar to see all the choices.) Explanation Check 10 (choose one) (choose one) linear bent T-shaped trigonal planar trigonal pyramidal square planar square pyramidal tetrahedral sawhorse trigonal bipyramidal octahedral Q Search 0/3 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center www.OTAPETY Accessibiarrow_forwardWhich of the following statements is true about ClF2–? Select one: The real bond angles are equal to the ideal bond angles. It is polar. It has a linear electron group geometry. The formal charge on the Cl is zero. It has a VSEPR class of AX3E2. Clear my choicearrow_forwardCalculate the number of valence electrons Molecule # 5 SiF?- Draw the Lewis Structure below Draw the three-dimensional structure of the molecule using the "wedge and dash" notation. The molecular geometry is: This molecule is polar nonpolar (Circle your choice.) Our logic for this choice is: The formal charge on Si is The formal charge on F is The hybridization of Si is Estimate F-Si-F bond angle Molecule # 6 CC2F2 Draw the Lewis Structure below Calculate the number of valence electrons Draw the three-dimensional structure of the molecule using the "wedge and dash" notation. The molecular geometry is: This molecule is polar nonpolar (Circle your choice.) Our logic for this choice is: The formal charge on C is The formal charge on Cl/F is The hybridization of C is Estimate F-C-Cl bond anglearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
