
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
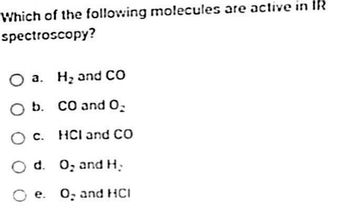
Transcribed Image Text:Which of the following molecules are active in IR
spectroscopy?
O a. H₂ and CO
O b. CO and O₂
O c. HCI and CO
Od. O₂ and H₂
Oe. O, and HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following has the largest CN stretching energy? OA.A OB. B OC.C O D.D Ο Ε.Ε C O NH₂ IZ A O D N B EN O E ZI Narrow_forwardWhat diene and dienophile would react to give the following Diels-Alder product? Select one: 3 CN A. Vand VI B. Ill and VI C. I and IV D. II and IV E. III and IV CN H CN III VI 0 ON Aarrow_forwardRank the basic properties of the following compounds by explaining the factors that influence them: NH3, PH3 dan AsH3 Pyridine, 2-methyl pyridine, 3-chloro pyridine NH3, NCI3 dan NF3 a. b. С.arrow_forward
- Which of the following molecules is least acidic? A OE O B O O C A B C D Earrow_forwardChoose the group that matches the following description: reacts with F2 to form compounds with the general formula MF2. Select one: a. Group 1A b. Group 2A c. Group 3A d. Group 4A e. Group 5Aarrow_forward4. Which of the following molecules would react with hydroxide and favor the formation of products? Explain your reasoning. a. b. NH3 C. OH 18 d. SHarrow_forward
- Chem 222 Recitation activity Chapter 14 mos 2 Name 1. For the penta-1,3-denyl anion, draw the molecular orbitals and the MO diagram. Identify the orbitals as bonding, non-bonding, or antibonding. Label the HOMO and the LUMO. (14.30) Please explam clearly how to determine how many molecular ORbitals get arrowso Thanksarrow_forwardWhich is the least activated towards EAS NO₂ A. B. CI C. D. CF3 E.arrow_forward4. Predict the spectral features for the following isomers of CsH₁0 and match each structure to a spectrum below. barrow_forward
- Bon and u Which of the following is NOT an organometallic compound? I B Select one: A. III B. IV CC. I 0.0 :O: Li II IV GHLI OCH,arrow_forwardIn IR spectroscopy, the energy absorbed by the molecule causes: A. In plane Bending B. Bond Vibration C. Electron Excitation D. Fragmentation O A & O A & D B&Carrow_forwardThe infrared spectrum of CBr4 has a strong absorption at 667 cm-1. What is the correct assignment of this absorption?and why ? a.) A bending or bending mode. b.) The asymmetric stretching of the four C-Br bonds. c.) The symmetric stretching of the four C-Br bonds. d.) The stretching of a C-Br bond.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY