
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
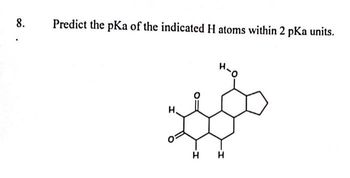
Transcribed Image Text:8.
Predict the pKa of the indicated H atoms within 2 pka units.
H
H-O
H H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate a K-Ar date for a sample of biotite from a gneiss containing K2O =9.00 wt%, 40Ar=2.424 mg/g. Decay constants: le = 0.581 x10-10a-1; l = 5.543 x10-10a-1; atomic abundance of 40K = 0.01167%; atomic weights: K=39.098, O= 15.999, 40Ar =39.96. And assume no inherited 40Ar.arrow_forwardCHEMICAL REACTIONS Soad V Balancing chemical equations with interferin... CH, CH, (g) + 0,(g) - co,(g) + H,0(g) ロ→ロ olo Ararrow_forward2. For pH values of 2.00, 6.00, and 10.00, calculate the alpha values for each species in an aqueous solution of phosphoric acid. ESarrow_forward
- Given the information about the following acids:i= HW pKa = 2ii= HX pKa = 6iii= HY pKa = 10iv= HZ pKa = 20Which these acids will react almost completely with hydroxide ion to form water?arrow_forwardChemical formula of Mg2+ and CO32-arrow_forwardWhy Potassium cyanide (KCN) is added in the limit test of lead?arrow_forward
- Butyric acid, the compound responsible for the unpleasant odor and taste of sour milk, has a pKa value of 4.82. What is its Ka value? Is it a stronger acid or a weaker acid than vitamin C?arrow_forwardHow do we find x? I kept getting 2 moles of H and 1 mole O but I know x is supposed to equal 2, so how do we get this?arrow_forwardThe Mohr's salt sample is diluted using a 0.5 M H,So,/ H;PO, solution. The function of the sulfuric acid is: Seleccione una. provide the protons for the reaction oxidize the ferrous ion reduce the chromic ion make the final point region more drastic change color when reaching the titration end pointarrow_forward
- 39arrow_forwardWhat chemical is described as 1MH2SO4?arrow_forwardDihydrogen phosphate, H₂PO₄⁻(aq), undergoes the following acid base equilibria in blood: H₂PO₄⁻(aq) ⇌ H⁺(aq) + HPO₄²⁻(aq)⇌ H⁺(aq) + PO₄³⁻(aq). The pKa values for the first and second steps are 7.2 and 12.4, respectively. The pH of human blood is 7.37 and this is tightly regulated. Which of the following statements most accurately describes the concentrations of the different species for the above reactions in blood. (Hint, think of the titration curves for H₂PO₄⁻(aq) and HPO₄²⁻(aq) and their respective pKa's.) A) [H₂PO₄⁻(aq)] is much greater than [HPO₄²⁻(aq)] and [PO₄³⁻(aq)] B) [HPO₄²⁻(aq)] is much greater than [H₂PO₄⁻(aq)] and [PO₄³⁻(aq)]. C) [H₂PO₄⁻(aq)] and [HPO₄²⁻(aq)] are both present and greater than [PO₄³⁻(aq)]. D) [PO₄³⁻(aq)] is much larger than the other concentrations, [H₂PO₄⁻(aq)] and [HPO₄²⁻(aq)]arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY