
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Which of the following is TRUE, if the protein pH is electrically neutral? Refer to the graph below.
a. The denaturation of protein is lower than 100%
b. The denaturation of protein is greater than 100%
c. The protein is in native form
d. The protein is completely denatured
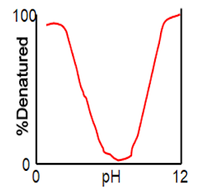
Transcribed Image Text:100
pH
12
%Denatured
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Which types of amino acids (polar, nonpolar, or charged) are expected to be found primarily in the core of proteins that are in aqueous solution? Justify your response.arrow_forwardc) Jean-François is studying a specific protein in a research laboratory. In previous experiments, he observed that this protein seems to function as a receptor protein on the surface of cells that recognizes a specific hormone involved in cell-cell communication. Jean-François prepares his final experiment, but makes a mistake in calculating how much HCl to add to one of his solutions to get the correct pH. He performs the experiment, and much to his surprise, he observes that his protein no longer seems to be recognizing the hormone anymore as he had observed in previous experiments. Propose a hypothesis that briefly explains (2-3 sentences) why the results of Jean-François’ experiment have changed. oportmost likely h beens dy the celyd must irst ir o nm cof the st omce MAnoncalest wanti patient Hewe umourcells Becnuse of falla anan intoindidual.cells. protein d) True or false: A mutation could cause the same change in Jean-François' experimental results as the change in pH described…arrow_forwardExplain why unfolding or aggrrgation may occur using changes in entropy and enthalpy of the protein and water. a. The shift of a pH from pH 7 to pH 11 b. Addition of Triton-x (detergent) into buffer c. Raising the temperature of the solution from 25 C to 100 Carrow_forward
- When a protein is denaturated, what happens to it?Describe two causes of denaturation.arrow_forwardAnswer the following multiple choice questions, and pick from the following a, b, c, darrow_forwardA purified protein fraction has a total sample volume of 360 µL. The sample has a corrected A280 of 0.484, and the blank corrected A280 was 0.052. (Both values were measured with a path length of 1.00 cm.) If 5.00 µL of the sample was used in a reaction, calculate the mass of protein in the reaction (in µg).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON