
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
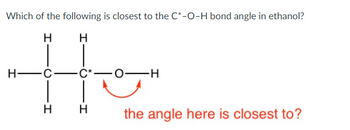
Transcribed Image Text:Which of the following is closest to the C*-O-H bond angle in ethanol?
Н
Н
He
H-C-
H
C*-
O- -H
H the angle here is closest to?
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- what is the bond angle for S - O - H in H2SO4?arrow_forwardFill in the bond angles for the molecules shown. D O-C-O bond angle = O || H-C-OH (b) CH3-O-CH3 C-O-C bond angle =arrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name dimethyl ether iodomethane acetic acid compound formula or Lewis structure H T H-C-Ö-C-H I H HILIA CH₂I | H H :O: | || H-C-C-O-H Between molecules of the compound? O yes O no O yes O no hydrogen-bonding force O yes O no Between molecules of the compound and molecules of water? O yes Ono O yes O no O yes O no X Sarrow_forward
- Choose the correct resonance hybrid for the following compound. 0 O O 49 -O-H ●ㅗㅗ 8+ 8+ HO-H co ---- 8+ 8+ H ○- + DHE O-H 8+ 8-Harrow_forwardThe molecular geometry of the right-most carbon atom in the molecule below is H- H H O Harrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Indicate the electron pair geometry and the molecular geometry for each of the six compounds. Compound :0=0-0: :C-S-CI: :CI-Be-Ci: CI-S-CI 0: :0=s=0: 0: H H-C-H 1 H ||||||| Electron pair geometry Molecular geometryarrow_forwardDraw the wedge and dash bond-line structurearrow_forward4. Convert the following structures into bond line structures. C-H ннн нон нн Н-с-с-с-о-н Н-с-с-с—Н Н-с-с-о-с-н H H H H H H нн H C. H- H-N H Ó H il н-с-с-с-с-н H H H H ннarrow_forward
- a Use the VSEPR model to predict the bond angles and geometry about each highlighted atom: HH H-C-C-O-H II HH b C about C 109.5°, tetrahedral; about O 109.5°, bent about C 109.5°, tetrahedral; about O 180°, linear about C 120°, planar; about O 109.5°, tetrahedral about C 109.5°, bent; about O 180°, linear HH H-C-C-H I HH 109.5°, tetrahedral 120°, planar 180°, linear 109.5°, bent HO H-C-C-C-H H 120°, planar 109.5°, tetrahedral 109.5°, bentarrow_forwardWhich of these is a proper Lewis structure of propylene, C3H6, in which all of the carbons are linear? H H H-C-C-C-H H H :H: H: :H: :C-C-C: •H: :H: •H H-C-H H-C-H HIC-H Н H-C=C=C-H HA H-C-C=C H HH Harrow_forwardFormula Lewis Electron Molecular Bond Polar or Attractive Structure Pair Geometry Angle Force Non Polar Geometry Between Molecules CH4 Around First C Around First C C2H4 Around First C Around First C C,H2 Around First C Around First C CH;OH Around O Around O C2H;OH Around O Around O CH20 CH,OCH, Around O Around O CH;COOH Central C Central C НСООН Central C Central C CH;NH2 Around N Around N CH;COCH; Central C Central Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
