
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
a) Which of the following is a key intermediate for Step 1 of the reaction shown in the box? (image 1)
b) please name the
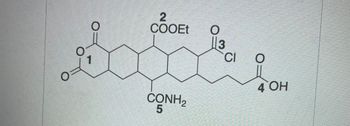
Transcribed Image Text:O
O
1
2
COOEt
CONH₂
5
O
||3
CI
O
From
4 OH
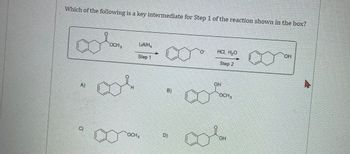
Transcribed Image Text:Which of the following is a key intermediate for Step 1 of the reaction shown in the box?
A)
C)
OCH,
LAIH,
Step 1
OCH,
B)
D)
HCI, H₂O
Step 2
OH
00%.
OCH,
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7) Assign partial charges on the Cl-Cl (polarized) below. This reaction proceeds through a three-membered ring halonium intermediate. The first step involves arrows similar to the carbene reaction above plus an additional arrow showing the Cl-Cl bond breaking. The second step is SN2 at the more substituted carbon. 8) Complete the following reaction by adding curved arrows, intermediates, and the expected products. St r :CI-CI: :CI: 9) Label the Mechanistic steps above and the halonium ion intermediate. Because the second step is similar to SN2 the halides add anti to each other.arrow_forwardOn the molecules on the back page, circle and label all of the functional groups that you see (identified in the table above). Please note the following tips that may help you on this assignment: If a molecule contains –COOH, or a C double bonded to an O and single-bonded to an –OH, the functional group you circle is only the carboxyl, not a carboxyl, carbonyl, and hydroxyl separately). Phosphate groups are tricky – any P surrounded by 4 O’s is a phosphate – adjacent phosphates may share an O, and sometimes the O is in the form of OH. There is often shorthand that is used in drawing these structures – if you see a jagged line or ring structure, assume that carbon is at each corner. There may be ionized forms of the functional groups above (such as NH2 or NH3+) – these are the same functional groups. The order of the atoms in the functional group may be switched, depending on which side of the molecule it is on. For example, “—NH3” and “H3N—“ are the same thing.arrow_forwardDraw both resonance structures of the mnost stable carbocation intermediate in the reaction shown +. HBr • You do not have to conside stereochemistry Do not include anionic counter-1ons, e g., I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu the bottom right corner. Separate resonance structures using the symbol from the drop-down menu. inarrow_forward
- A=B When a curved arrow starts from an bond and points to an adjacent atom, the n bond breaks and the o bond remains. This will typically generate two charges. A-B=C Note in the second mechanism shown, if the first curved arrow started from atom C, then atom B would violate the octet rule. A second a bond would need to delocalize. Read the curved arrow in the mechanism shown and draw the product. Be sure to draw lone pairs. Select Draw Rings More Erase C Harrow_forwardDraw all significant resonance structures for the following compound: Step 1 First, add curved arrow(s) to show the resonance using the following pattern: an allylic carbocation. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to increase or decrease the charge on an atom, and use the single bond tool to add/remove double bonds.arrow_forward5. Using curved arrows show the mechanism for the hydrohalogenation for the following reaction. Show how the two possible intermediate products are formed and explain why only one product proceeds to form the product. Clearly indicate attack of electrons and full and partial (8) charges. H-Br Br H-Br Product Intermediate Productsarrow_forward
- Please don't provide handwritten solution ....arrow_forwardOn the molecules on the back page, circle and label all of the functional groups that you see (identified in the table above). Please note the following tips that may help you on this assignment: If a molecule contains –COOH, or a C double bonded to an O and single-bonded to an –OH, the functional group you circle is only the carboxyl, not a carboxyl, carbonyl, and hydroxyl separately). Phosphate groups are tricky – any P surrounded by 4 O’s is a phosphate – adjacent phosphates may share an O, and sometimes the O is in the form of OH. There is often shorthand that is used in drawing these structures – if you see a jagged line or ring structure, assume that carbon is at each corner. There may be ionized forms of the functional groups above (such as NH2 or NH3+) – these are the same functional groups. The order of the atoms in the functional group may be switched, depending on which side of the molecule it is on. For example, “—NH3” and “H3N—“ are the same thing.arrow_forwardQuestion is in photoarrow_forward
- Need to check answer 1.Borane (BH3) adds to alkenes to form an alkylborane. In the first box draw the mechanism arrows, and in the second box draw the correct product. Be sure to add lone pairs of electrons and nonzero formal charges to all species.arrow_forwardNitesharrow_forwardPredict the major products of this organic reaction. Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY