
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
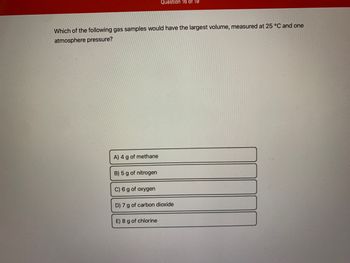
Transcribed Image Text:Which of the following gas samples would have the largest volume, measured at 25 °C and one
atmosphere pressure?
A) 4 g of methane
B) 5 g of nitrogen
Question 16 of 19
C) 6 g of oxygen
D) 7 g of carbon dioxide
E) 8 g of chlorine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assume the molecular mass of air to be 28.5 g/mol. How many moles of air are above 1 square inch of sea level (all the way to outer space)?arrow_forwardHow many liters of oxygen gas are needed to react with 1.3 x10 23 molecules of glucose?arrow_forwardWhat volume of N2, measured at 24 °C and 719 mm Hg, will be produced by the decomposition of 14.5 g NaN3?2 NaN3(s) 2 Na(s) + 3 N2(g)arrow_forward
- how many moles of S2 are needed to produce .750 moles of SO2 gas?arrow_forwardA sample of hydrogen gas collected at a pressure of 0.906 atm and a temperature of 12.0 °C is found to occupy a volume of 23.6 liters. How many moles of H₂ gas are in the sample? molarrow_forwardA sample of hydrogen gas collected at a pressure of 0.806 atm and a temperature of 6.00 °C is found to occupy a volume of 21.2 liters. How many moles of H2 gas are in the sample? molarrow_forward
- A sample of polyatomic gas weighs 6.94 g and at a temperature 30.0 C it has a volume of 2.49 L and a pressure of 361 torr. What is the molecular weight of the gas? (hint: remember that PV=nRTPV=nRT) The molecular structure of the gas in the above problem has a total of 7 atoms and contains only sulfur and fluorine atoms. Based on its molecular weight you calculated above, which of the following empirical formulas is most likely to be correct for the gas? SF6 S2F5 S3F4 S4F3 S5F2 S6F None of these formulas are correctarrow_forwardHow many argon atoms are contained in 7.66 x 105 mmol of argon? 4.61 x 1026 Ar atoms 1.84 x 1028 Ar atoms 1.15 x 1028 Ar atoms 7.86 x 1020 Ar atoms O 3.24 x 1026 Ar atomsarrow_forwardWhat volume of hydrogen gas will be produced when 5.00 g of calcium reacts with excess hydrochloric acid at STP? (STP is standard temperature and pressure. Standard temperature is 0oC and standard pressure is 1.00 atmospheres.) What is the name of this law?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY