
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
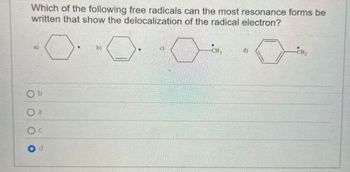
Transcribed Image Text:Which of the following free radicals can the most resonance forms be
written that show the delocalization of the radical electron?
Ob
O a
Od
b)
CH₂
d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Formamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardWhat is the formal charge on the indicated atom in each of the following species? (a) sulfur in SO2 (b) nitrogen in N2H4 (c) each oxygen atom in ozone, O3arrow_forwardWhat is the resonance form that describes the distribution of electrons in SeO2 ?arrow_forward
- Arrange the following radicals in order of increasing stability, unut Oarrow_forwardIn the following compounds, the C atoms form a single ring.Draw a Lewis structure for each compound, identify cases for which resonance exists, and determine the carbon-carbon bondorder(s): (a) C₃H₄; (b) C₃H₆; (c) C₄H₆; (d) C₄H₄; (e) C₆H₆.arrow_forwardalculate the formal charge (example +1) of chlorine in the most stable form of the perchlorate anion (Hint: draw its Lewis structure first):arrow_forwardDraw all the equivalent resonance structures for BrO 3¯ on a piece of paper and then fill in the blanks. Draw the structure in such a way that the formal charge on the central atom is zero. Molecular geometry: Number of equivalent resonance structures: Bond order of Br-O bond(s): (fractions rounded to 2 decimal places, e.g. 1/2 will be 0.50 , 4/3 will be 1.33 and 5/3 will be 1.67) Formal charge on terminal atoms participating in resonancearrow_forwardThe hydrocarbon cyclobutane, C4H8, is represented above. At high temperatures, cyclobutane quickly decomposes into ethene, C2H4. (see attached image) (a) Draw a Lewis electron-dot diagram of the ethene molecule in the following box, and estimate the value of the H−C−H bond angle in ethene.arrow_forwardDetermine the formal charge on each atom in the ion H3O+ ?arrow_forwardHow many resonance structures can be drawn for the dehydrogen antimonate ion (H2SbO4 -) in which the central antimony atom bears a -1 formal charge and the oxygens bear formal charges of either zero or -1?arrow_forwardWhich of the following is the correct bond-line structure for (CH3)4C? I OI O II O III OIV None of these II x III IVarrow_forwardSuppose that any given kind of bond, such as 0-H, has a characteristic electric dipole. That is, suppose that elec- tric dipole moments can be assigned to bonds just as bond energies can be. Both are usefully accurate approxi- mations. Consider the water molecule H `H Show that if MOH is the dipole moment of the OH bond, then the dipole moment of water is µ(H2O) = 2µ0H cos (0/2). What is the dipole moment µoH if µ(H,O) is 1.86 D?arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning