
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
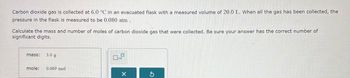
Transcribed Image Text:Carbon dioxide gas is collected at 6.0 °C in an evacuated flask with a measured volume of 20.0 L. When all the gas has been collected, the
pressure in the flask is measured to be 0.080 atm.
Calculate the mass and number of moles of carbon dioxide gas that were collected. Be sure your answer has the correct number of
significant digits.
mass: 3.0 g
mole: 0.069 mol
ㅁ
x10
x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider these four gas samples, all at the same temperature. The larger boxes have twice the volume of the smaller boxes. Rank the gas samples with respect to: (a) pressure, (b) density, (c) average kinetic energy, and (d) average molecular speed. (Green spheres are He; violet spheres are Ne.)arrow_forward109 An ore sample with a mass of 670 kg contains 27.7% magnesium carbonate, MgCO3. If all of the magnesium carbonate in this ore sample is decomposed to form carbon dioxide, describe how to determine what volume of CO2 is evolved during the process. What would have to be measured to predict the needed volume in advance?arrow_forwardA balloon filled with helium gas is found to take 6 hours to deflate to 50% of its original volume. How long will it take for an identical balloon filled with the same volume of hydrogen gas (instead of helium) to decrease its volume by 50%?arrow_forward
- Two identical He-filled balloons, each with a volume of 20 L, are allowed to rise into the atmosphere. One rises to an altitude of 3000 m while the other rises to 6000 m. a Assuming that the balloons are at the same temperature, which balloon has the greater volume? b What information would you need in order to calculate the volume of each of the balloons at their respective heights?arrow_forwardNitrogen monoxide gas reacts with oxygen gas to produce nitrogen dioxide gas. What volume of nitrogen dioxide is produced from the reaction of 1 L nitrogen monoxide gas with 3 L oxygen gas? What volume, if any, of the reactants will remain after the reaction ends? Assume all volumes are measured at the same pressure and temperature.arrow_forwardYou have a gas, one of the three known phosphorus-fluorine compounds (PF3, PF3, and P2F4). To find out which, you have decided to measure its molar mass. (a) First, yon determine that the density of the gas is 5.60 g/L at a pressure of 0.971 atm and a temperature of 18.2 C. Calculate the molar mass and identify the compound. (b) To check the results from part (a), you decide to measure the molar mass based on the relative rales of effusion of the unknown gas and CO2. You find that CO2 effuses at a rate of 0.050 mol/min, whereas the unknown phosphorus fluoride effuses at a rate of 0.028 mol/min. Calculate the molar mass of the unknown gas based on these results.arrow_forward
- Helium-filled balloons rise in the air because the density of helium is less than the density of air. (a) If air has an average molar mass of 29.0 g/mol, what is the density of air at 25C and 770 mm Hg? (b) What is the density of helium at the same temperature and pressure? (c) Would a balloon filled with carbon dioxide at the same temperature and pressure rise?arrow_forwardDichlorine oxide is used as bactericide to purify water. It is produced by the chlorination of sulfur dioxide gas. SO2(g)+2Cl2(g)SOCl2(l)+Cl2O(g)How many liters of Cl2O can be produced by mixing 5.85 L of SO2 and 9.00 L of Cl2? How many liters of the reactant in excess are present after reaction is complete? Assume 100% yield and that all the gases are measured at the same temperature and pressure.arrow_forwardThe atmosphere in a sealed diving bell contained oxygen and helium. If the gas mixture has 0.200 atm of oxygen and a total pressure of 3.00 atm, calculate the mass of helium in 10.0 L of the gas mixture at 40C.arrow_forward
- 94 Mining engineers often have to deal with gases when planning for the excavation of coal. Some of these gases, including methane, can be captured and used as fuel to support the mining operation. For a particular mine, 2.4 g of CH4 is present for every 100.0 g of coal that is extracted. If 45.6% of the methane can be captured and the daily production of the mine is 580 metric tons of coal, how many moles of methane could be obtained per day?arrow_forwardA chemist weighed out 5.14 g of a mixture containing unknown amounts of BaO(s) and CaO(s) and placed the sample in a 1.50-L flask containing CO2(g) at 30.0C and 750. torr. After the reaction to form BaCO3(s) and CaCO3(s) was completed, the pressure of CO2(g) remaining was 230. torr. Calculate the mass percentages of CaO(s) and BaO(s) in the mixture.arrow_forward82 Why do heavier gases move more slowly than light gases at the same temperature?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning