
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question
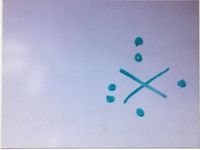
Which of the following elements has this electron dot diagram
a) oxygen, sulfur
b) magnesium, calcium
c) lithium, flourine
d) none of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Draw the electron dot formula for the following elements. (a) C. (b) He (c) Alarrow_forwardIllustrate the sharing of electrons of each covalent compound using the Bohr Model. Apply the octet rule if possible. (A) A molecule composed of two chlorine atoms. (b) A molecule composed of a hydrogen atom and a bromine atom. (c) A molecule composed of 2 hydrogen atoms. (d) A molecule composed of a hydrogen atom and a chlorine atom.arrow_forward(a) Group 1A positive + The elements in group 1A configuration. an electron (obtain a charge) in order to achieve a noble gas ...arrow_forward
- Give the ground-state electron configuration for each of the following elements. (a) Nitrogen (b) Heliumarrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardDisscuss why technetium and promethium have whole number atomic masses and have a bracket symbol on the periodic table.arrow_forward
- Which one in each pair has the larger radius ? Explain. (a) a magnesium ion or an aluminum ion (b) a sodium atom or a silicon atom (c) a potassium ion or bromide ionarrow_forwardState the predicted ionic charge of nonmetal ions in each of the following groups of elements. (a) Group IVA/14 (b) Group VA/15 (c) Group VIA/16 (d) Group VIIA/17arrow_forwardPlease answer the question below in DETAIL.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning