
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
3
Transcribed Image Text:1
4
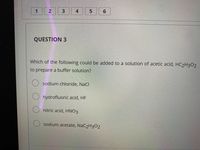
QUESTION 3
Which of the following could be added to a solution of acetic acid, HC2H3O2
to prepare a buffer solution?
O sodium chloride, NaCl
O hydrofluoric acid, HF
O nitric acid, HNO3
sodium acetate, NAC2H302
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are advantages and limitations of different types of modelling? (modelling with experiments, computer simulations, modelling kits and quantitative modelling)arrow_forwardQ1. The net potential energy between two adjacent ions, EN, is represented by A В E N pn where EN is the net energy in Joule (J), A and B are constants with values of 1.436 J.nm and 7.32 × 106 J.nm, r is the interatomic distance between the two ions in nanometer (nm), and n is a positive number with a value of 8.arrow_forwardWhich piece of equipment would you use to measure 7.10 mL of CuSO4 (aq)?arrow_forward
- Scientists can learn a great deal about a community's drug use (both legal and illegal) by analyzing the contaminants in wastewater. In a recent study in New York State, chemists measured the concentration of cotinine, a derivative of nicotine that i produced in the liver and excreted in the urine of tobacco users. The chemists found the average cotinine concentrations in the wastewater stream was 1.43 µg/L. a Ι Ι H- b HH H H-C C-N: C=0: TH HH Jonutis/Shutterstock Revell, Introductory Chemistry, 2e, © 2021 W. H. Freeman and Company Cotinine is slightly soluble in water. Using the structure of cotinine, identify any polar covalent bonds that are present in this substance. C-O C-C C-H C-Narrow_forwardча OH A OH D B с CHOarrow_forwardThe world population is estimated to be 7.4 x 109. Nauru is the smallest island nation and comprises 1.5 x 10-4% of the world population. If the percentage of left-handed people is approximately 12%, estimate the number of left-handers on the island of Nauru.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY