
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
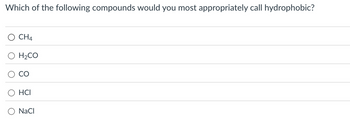
Transcribed Image Text:Which of the following compounds would you most appropriately call hydrophobic?
○ CH4
H2CO
CO
HCI
○ NaCl
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- What are the 5 functional groups?arrow_forwardFor each pair of compounds, select the compound that would be more soluble in water. Explain your answer briefly. You may refer to the following electronegativity values. F= 4.0; I=2.4; N= 3.0; O=3.5; H=2.1; As=2.0; S=2.5a. C2H5F and C2H5Ib. C3H7OH and C3H7SHc. NH3 and AsH3arrow_forwardYour roommate, a chemistry major, claims to have synthesized the compound CH5 in the lab. Why is that not possible?arrow_forward
- How many electron pairs are shared when a triple bond exists between two carbon atoms? What must he the geometric arrangement around the carbon atoms in a triple bond? Draw the Lewis structure of a simple molecule that contains a triple bond.arrow_forwardNucleotides are molecules that make up the structures of RNA and DNA. Modify the compounds to create adenosine monophosphate (AMP).arrow_forwardList the functional groups in the structures below:arrow_forward
- We see that 1-propanol and 2-propanol have the same molecular formula, C3H7OH, but different molecular structures. What is the name for molecules that have the same molecular formula but different structural formulas (different shapes)? Use the specific term.arrow_forwardFor each pair of compounds, select the compound that would be more soluble in water. Explain your answer briefly. You may refer to the following electronegativity values. F=4.0; I=2.4; N=3.0; 0=3.5; H=2.1; As=2.0; S=2.5 a. C2H5F and C2H5I b. C3H7OH and C3H7SH c. NH3 and ASH3 d.arrow_forwardWhich of the following does not form H-bonding with water? Propyne CHF3 CH3CN dimethylaminearrow_forward
- For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. compound hydrogen-bonding force Between Between molecules of formula or Lewis the compound and molecules of water? name molecules of the structure compound? H yes yes | H – c=N– H methanimine no no H yes Н —с —N— н | methylamine no yes yes dichloromethane CH, Cl, no no O O H- Z:arrow_forwardThe element which causes the greatest polarity within an organic molecule is ___. oxygen nitrogen carbon hydrogenarrow_forwardWrite the common (not systematic) name of each organic molecule. structure IN name ☐ | 5 10 000 ☐ Ar لكاarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning