
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
6
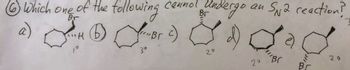
Transcribed Image Text:6 Which one of the following
46 Br
ioH
3°
سے
cannot undergo an S2 reaction?
20
20
AR(
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3* 6. What element corresponds to 1s22s22p63s23p64s23d104p6? 7. Correct the errors in the following: 1s22s32p1 1s22s22p63s23d8 1s32s22p63s23p54s1 1s22s22p63s23p63d7arrow_forwardis this right?arrow_forwardデジタル形式で段階的に解決 ありがとう!! SOLVE STEP BY STEP IN DIGITAL FORMAT Structure of Metal Materials: 8. A colleague calculated the density of iron with BCC structure using the following data of atomic radius equal to 1.24 Angstrom and atomic weight of 55.85 as follows: p = [(4 atoms / C. U.) x (55.85 g/atomic weight)/(atoms/atomic weight) / [2(1.24x108 cm)/√2]³ p = 68.8 g/cm³ Is the result obtained logical? Explain and justify your answer.arrow_forward
- 2.5 x 3.42. The answer to this problem would be 8.55 Select one: O True O Falsearrow_forwardHelp 100% 4A To Public Health Ch x * HSC 258 - Major Projec X MindTap - Cengage Lea X C The Illustration To TH d%=55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search th References Use the References to access important values if needed for this question. For the following reaction, 24.7 grams of sulfur dioxide are allowed to react with 9.95 grams of oxygen gas . sulfur dioxide(g) + oxygen(g) → sulfur trioxide(g) What is the maximum mass of sulfur trioxide that can be formed? grams What is the FORMULA for the limiting reagent? grams What mass of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardLead storage batteries are used in car engines. Group of answer choices True Falsearrow_forward
- You have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C34H32FEN.O4, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 10.9 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample?arrow_forward(b) 0.74 MMgSO4 lons: So,2- O So,- SO- Sot Mg2+ Mg* 04 [cation*] = %3D M [anion]= M %3Darrow_forward(256) Malang Sajna (Video) S Significant Figures Calculator - Six Type here to search www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnuspTU6txxTOZ396sp-B_xkmco0cVWd500mjk4S42nF8fdH9EGW-KtycTAasP6... Q ● ENTROPY AND FREE ENERGY Calculating dG from dH and dS 4PF, (g) + 10H₂(g) P4 (s) + 20HF (g) A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 53.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. CH₂(g) + 20₂(g) → CO₂(g) + 2H₂O(g) ALEKS - Rafia Riaz - Learn Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then,…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY