
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
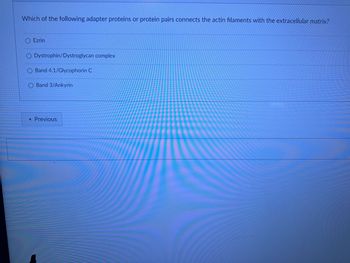
Transcribed Image Text:Which of the following adapter proteins or protein pairs connects the actin filaments with the extracellular matrix?
Ezrin
Dystrophin/Dystroglycan complex
Band 4.1/Glycophorin C
O Band 3/Ankyrin
< Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Which of the following proteins is categorized as a single-pass transmembrane protein (containing the blood-typing carbohydrate antigens of the human ABO system)? band 3.0 protein (in the red blood cell membrane) COX-1 (in the lumenal leaflet of the smooth endoplasmic reticulum) glycophorin A (in the red blood cell membrane) the Ras protein (lipid-linked to the inner plasma membrane) cytochrome c (in the mitochondrion)arrow_forwardWhat is meant by the term "Critical concentration" with respect to actin filaments?arrow_forwardSequence: AAA UGG CAA Translate the sequence into the correct amino acids. Question 2 options: Lys Trp Stop Lys Trp Gln Stop Lys Stoparrow_forward
- When nerve cells contact each other, they form adherens junctions (AJs) at the point of contact. The main transmembrane (TM) protein in the junctions is N-cadherin. N-cadherin is a single pass TM protein with a short intracellular domain and a large extracellular domain on the amino end. Proper formation of the AJs requires both actin (MF) and microtubules (MTs). Motor proteins for MTs are dynein (-) end and kinesin (+) end (and were discovered by Michael Sheetz). Maintenance of preformed AJs requires MFs . Adding drugs that disrupt MFs causes AJs to fall apart, and the N-cadherin is removed from the PM (by endocytosis). If the drugs are removed, MTs (& MFs) are required to restore the N-cadherin to the plasma membrane. choose answer for D1 and D2 D. Suppose cells contain a soluble kinase that modifies N-cadherin that has been removed from the PM. D-1. The kinase could catalyze modification(s) of the side chains of amino acids near (the N end) (the C end) (either end, but not…arrow_forwardYou coat microfilaments with the myosin S1 fragment and add these coated microfilaments to the slide with Protein Y stuck on a slide. You observe the coated microfilaments move, and they move in the direction with the barbed ends leading. Your advisor is ecstatic with this observation, telling you that you have made a major discovery. What is novel about this discovery? ng. Microfilament movement Protein Yarrow_forwardWhy is the proportion of actin within filaments in cells smaller than would be predicted by in vitro experiments?arrow_forward
- The synaptosome-associated receptors referred to as v-SNARES are transmembrane proteins located on which of the following eukaryotic cell structures? mitochondrial membranes chloroplast membranes vesicle membranes lysosomal membranes peroxisomal membranesarrow_forwardThe difference in microtubule bundles stabilized by Tau and MAP2 is due to differences in amino acid sequences in the the tubulin subunits whether or not the alpha subunit of tubulin is bound by GTP or GDP the size difference between Tau and MAP2 the phosphorylation state of Tau and MAP2arrow_forwardWhat is a common feature of the voltage sensitive Ca++, Na+ and K+ ion channels? A single polypeptide sequence made up of 4 domains A voltage sensing S4 transmembrane domain A transmembrane S5-S6 hydrophobic subunit that binds the membrane A voltage sensing extracellular P looparrow_forward
- What key ATPase is important for maintaining this positive (outside) and negative (inside) transmembrane potential?arrow_forwardIdentify how each of the following would pass through the plasma membrane: gases, large polar molecules, charged molecules (like ions), hydrophobic molecules, and small polar molecules. Be specific.arrow_forwardThe following figure shows the 3D structure of a tubulin dimer. A mutation in the beta tubulin subunit (shown in red) prevents the binding of a GTP molecule. Predict the consequences that this mutation will have on the microtubule and on cell division. Please be specific. Alpha tubulin Beta tubulinarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education