
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
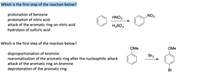
Transcribed Image Text:Which is the first step of the reaction below?
protonation of benzene
protonation of nitric acid
attack of the aromatic ring on nitric acid
hydrolysis of sulfuric acid
HNO3
ZON
H2SO4
Which is the first step of the reaction below?
OMe
OMe
disproportionation of bromine
rearomatization of the aromatic ring after the nucleophilic attack
attack of the aromatic ring on bromine
deprotonation of the aromatic ring
Br2
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the product of this reaction?arrow_forwardChoose the nucleophile best suited for the conversion of an acid chloride to a carboxylic acid. na NaOCH3 CH3NH2 H₂O CH3OH ? OHarrow_forwardChoose the best reagents to complete the reaction shown below. OH 00 OH OH Q Q A B C D NaOH LiF H₂O NaHarrow_forward
- propose mechanisms for the following reactions.arrow_forward33a, no need to explain too much.arrow_forwardPh- OC XT :OH₂ Primary amines add to aldehydes and ketones to give imines. Imines are formed in a reversible, acid-catalyzed process that begins with nucleophilic addition of the primary amine to the carbonyl group, followed by transfer of the proton to yield a neutral carbinolamine. Protonation of the hydroxyl group converts it into a good leaving group and an E1-like loss of water yields an iminium ion. Deprotonation yields the product imine and regenerates the acid catalyst. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions NHOH CH3 CH3 NH₂OH OH HN I Ph CH3 OH H₂O CH3 заarrow_forward
- What is the product of the reaction shown below? pyridine OHarrow_forwardA set of three nucleophilic displacement reactions is shown below: - Br -Br -Br "CHз Hзс CHз C В A NaCN CH-он SN2 reaction Which reaction (A, B, or C) proceeds the fastest? Which reaction (A, B, or C) proceeds the slowest?arrow_forwardWhat is the expected product for the following reaction sequence? CI H Ag₂O NaOH 27 Productarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY