
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
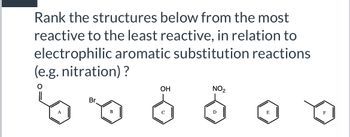
Transcribed Image Text:Rank the structures below from the most
reactive to the least reactive, in relation to
electrophilic aromatic substitution reactions
(e.g. nitration)?
A
Br
B
OH
NO2
E
F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 1 images

Knowledge Booster
Similar questions
- Rank the reactivity of the compounds below toward nucleophilic acyl substitution by writing the compounds' letters in the proper blanks in the box below. `NH CI Br CH3 CH3 A В C E rank compounds for acyl substitution reactivity most least reactive reactivearrow_forwardRank group of compounds from most reactive to least reactive toward electrophilic aromatic substitution: toluene, p-cresol, benzene, p-xylenearrow_forwardElectrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid, p-methoxybenzoic acid or p-(methylthio)benzoic acid? Explain.arrow_forward
- Rank the following carboxylic acid derivatives in decreasing order (most to least) of reactivity towards nucleophilic substitution CI NH2 OCH3 ONa II IV OI> IV> III > || O > IV > || >I O I> II| > || > IV O II > IV > 1> Iarrow_forwardArrange the structure on the image with regard to the reactivity towards nucleophilic acyl substitution 1 being the least and 3 being the mostarrow_forwardComplete the curved arrow notation and include the structure of the missing organic intermediate for the following steps of a nucleophilic aromatic substitution via an addition–elimination mechanism.arrow_forward
- Which of the following structures and IUPAC names are incorrectly matched? * oe (A) Methyleyclohexanoate (В) `NH2 Br 4-Bromo-3-chlorohexanamide 2-Ethoxypentane (D) HOOC HOOC 3,5-Nonanedioic acid В A Darrow_forwardProvide an arrow pushing mechanism for the following reactionarrow_forwardWhat is the first step in the mechanism for electrophilic aromatic substitutions and What is the driving force for losing a proton as the last step in electrophilic aromatic substitutions? Addition of the electrophile to the aromatic ring; Regeneration of the catalyst O Addition of the electrophile to the aromatic ring; Loss of the electrophile from the aromatic ring O Protonation of the aromatic ring, To make the ring more reactive O Generation of the electrophile; To restore an aromatic system O Deprotonation of the aromatic ring; To make room for the electrophilearrow_forward
- Consider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and D. (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major product(s) formed when this compound is treated with one equivalent of Br2?arrow_forwardPlese don't provide hand writtin solution....arrow_forward4) Rank the following compounds in order of increasing reactivity (least to most reactive) with respect to acyl substitution. I. methyl benzoate II. benzoylchloride III. benzamide III < | < ||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning