
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
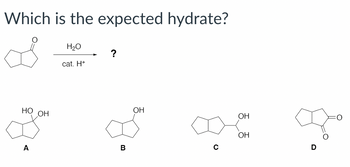
Transcribed Image Text:Which is the expected hydrate?
H₂O
cat. H+
?
НО ОН
ОН
A
B
о
ОН
ОН
Про
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 2 HC= CH + 5 O2 > 4 co2 + 2 h2o What is the energy of the reaction and is it exo- or endothermic?arrow_forwardCarbon forms a number of allotropes, two of which are graphite and diamond. Silicon has a diamond structure. Why is there of allotrope of silicon with a graphite structure?arrow_forwardWhich species have AH°; = 0? C (s, diamond) O Zn (s) O I2(8) O F2 (1) O Hg (s)arrow_forward
- stru Wharrow_forwardWill the following reaction occur as written? HO, H. H2N cat. H2SO4arrow_forward1) Choose the formation reaction for Li2SO4. A) 2 Li(s) + 1/8 S8(s, rhombic) + 2 O2(g) B) 16 Li(s) + S8(s, rhombic) + 16 O2(g) → C) L¡2SO4(aq) D) 8 L¡2SO4(s) E) 2 Li*(aq) + SO4² (aq) LiżSO4(s) 8 LizSO4(s) 2 Li*(aq) + SO,²-(aq) 16 Li(s) + S§(s, rhombic) + 16 O2(g) Lİ2SO4(aq)arrow_forward
- O Neither 8. What does the ion phosphate contain? * O 1 phosphorus, 4 oxygen, to the third power negative 1 phosphorus, 4 oxygen, to the second power negative O 2 phosphorus, 4 oxygen, to the third power negative O 1 phosphorus, 5 oxygen, to the second power negative 9 What is the difference between sulfate and sulfite? *arrow_forwardHow many kj of heat are needed to produce 6.47 g NH 3 ? 4NO+6H 2 O 4NH 3 +5O 2; triangle H=906 kJarrow_forwardAncient Romans built often out of bricks and mortar. A key ingredient in their mortar was quicklime (calcium oxide), which they produced by roasting limestone (calcium carbonate). 1. Write a balanced chemical equation, including physical state symbols, for the O-0 decomposition of solid calcium carbonate (CaCO2) into solid calcium oxide and gaseous carbon dioxide. da 2. Suppose 50.0 L of carbon dioxide gas are produced by this reaction, at a temperature of 400.0 °C and pressure of exactly 1 atm, Calculate the mass of calcium carbonate that must have reacted. Round your answer to 3 significant digits. 0g Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Accessibility MacBook Air DI 888 F9 F10 F7 F8 F3 F4 F5 F6 F1 23 2$ & の %24arrow_forward
- X 19. Calculate the mass of oxygen (in mg) dissolved in a 5.00 L bucket of water exposed to a pressure of 1.13 atm of air. Assume the mole fraction of oxygen in air to be 0.21 given that kH for O2 is 1.3 × 10-3 M/ atm at this temperature. 23.5 mg a b) 27.3 mg c) 49.4 mg d) 13.7 mg e) 9.87 mgarrow_forwardThe product(s) of the following reaction I equiv. HBr is: heat Br HO II HO HO Br O. III IV O I O IV O None of these choices.arrow_forward11) Adding sodium hydride (NaH) to water produces: (a) H2 and NaOH (b) H3O* and Na (c) H3O and Na* (d) Na20 and H2 (e) No reaction occursarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning