
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
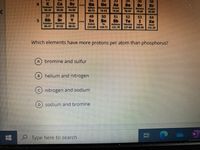
Transcribed Image Text:35
Ca
Caldum
40.08
Sc
Dondum
44.96
Ge
Cu Ceme
72.63
36
Kr
As
Se
Br
remine
79.90
39.30
0.72
74.92
78.97
37
39
38
Sr
49
In
ndum
114.62
50
Sn
51
Sb
imeny
121.76
52
Te
53
54
Rb
Rubidum Srotum
5.47
Xe
Xanon
131.29
...
rerlem
Teluum
127.60
Sodine
126.90
87.62
118.71
Which elements have more protons per atom than phosphorus?
bromine and sulfur
B) helium and nitrogen
nitrogen and sodium
D.
sodium and bromine
Type here to search
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the protons and electrons related in an atom that is neutralarrow_forwardThe number of protons determines the element. True O Falsearrow_forwardIf you know ONLY the following information, can you ALWAYS determine what the element is? Number of electrons Number of protons Number of neutrons Number of electrons in a neutral atom Select yes or no for each optionarrow_forward
- arrow_forwardRosa's unknown made-up compound contains element D and element Z. It has a molar mass of 1070 g/mol Analysis of a sample shows that it contains 8.41% element D and 91.59% element Z What is its molecular formula of the made-up compound? Please refer to the Mini Periodic Table of Fake Elements to answer your questions Mini Periodic Table of Fake Elements O DZ2 O D20 Z11 D3Z7 O D6Z₁4 DJ 15.00 Avogadro's Number: 6.022 x 1023 19.00 Q Z 35.00 70.00arrow_forwardIdentify recognise and name of subatomic particles of an atomarrow_forward
- IV. Supply the missing data in the table below. Use your periodic table of elements. Atomic Number Atomic Number of Number of Number of Mass Symbol Protons Electrons Neutrons Number 3274GE 32 32 74 37 37 36 49 8160 8 8 16 53 53 54 74 82 82 82 208arrow_forwardPlease use the options below to choose one correct answer to the question in the attached image. two more protons than carbon 12 two more electrons than carbon 12 two more neutrons than carbon 12 two more protons and neutronsarrow_forwardQUESTION 3 As a result of Aston's work, we know that two nuclei are isotopes when they have the same atomic weight but different atomic numbers the same atomic number but different atomic weight the same atomic number and the same atomic weight different atomic weights and different atomic numbersarrow_forward
- Based on the information below, what is the elemental symbol of this ion if it has 26 electrons?arrow_forwardPlease check and see if I got the information in the table below correct. If there are mistakes please correct them. Protons Neutrons Electrons Element Symbol Element Name Net Charge Mass Number Valence Electrons 1 0 1 H Hydrogen 0 1 1 6 6 6 C Carbon 0 12 4 8 8 8 O Oxygen 0 16 6 3 4 2 Li Lithium +1 7 0 5 6 4 B Boron +1 11 3 4 4 2 Be Beryllium +2 8 2 9 10 10 F Fluorine -1 19 8 2 2 2 He Helium 0 4 2 5 4 7 B Boron -2 9 5 6 6 6 C Carbon 0 12 4arrow_forwardWhy are atoms electrically neutral? O they have more electrons than protons O they have less electrons than protons they have the same number of electrons as neutrons they have the same number of electrons as protons O they have the same number of neutrons as protonsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY