
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
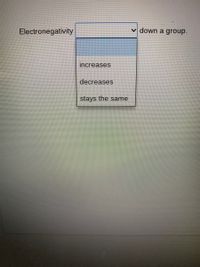
Transcribed Image Text:Electronegativity
v down a group.
increases
decreases
stays the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hydrochloric acid molecule holds together by a(n) O ionic bond polar covalent bond double bond nonpolar bond none of thesearrow_forwardIdentify the INCORRECT statement below: a) Electronegativity of an element is the measure of the relative tendency of an atom to attract electrons to itself which chemically combined. b) Electronegativity of metals is generally smaller than that for nonmetals. c) The element with the highest electronegativity is fluorine. d) Electron affinity is the energy required to attach an electron to a gaseous neutral atom. e) The electron affinity if fluorine is large and positivearrow_forwardPlease categorize the following bond based on its bond polarity Si-P? A)Pure covalent bond B)Polar covalent bond C)Ionic Bondarrow_forward
- Electronegativity V across a period. increases decreases stays the samearrow_forwardQUESTION 14 Rank the following in their electronegativity from most electronegative to least v Phosphorous v Potassium v Fluorine • Silicon v Lithlumarrow_forwardCircle all the true statements below: E) A covalent bond is found in non metal-non metals interactions F) An ionic bond is found in nonmetal-non metals interactions G) In a polar covalent bond electrons are shared but not equally H) In a non polar covalent bond electrons are transferred from one atom to the otherarrow_forward
- Which of the following best describes electronegativity trends in the periodic table?I. Electronegativity inreases from right to left across a period.II. Electronegativity inreases from left to right across a period.III. Electronegativity inreases from bottom to top within a group.IV. Electronegativity inreases from top to bottom within a group. Select one: a. I and III b. I and IV c. II and III d. II and IV e. None of these.arrow_forward1 Electrostatic attractions holding together oppositely charged ions is the definition of a a) polar covalent bond c) covalent bond b) ionic bond. d) municipal bondarrow_forwardIn an ionic compound: A. They share electrons equally B. They share electrons but the most electronegative atom "pulls" most of the electrons toward its side C. The electrons are not shared but each molecule keeps its electrons when they form the bond D. Electrons are not shared the most electronegative atoms(s) takes all the electrons from the metalarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY